|
ICI-164384
ICI-164384, also known as ''N''-''n''-butyl-''N''-methyl-11-(3,17β-dihydroxyestra-1,3,5(10)-trien-7α-yl)undecanamide, is a steroidal antiestrogen and a synthetic derivative of estradiol which is closely related to fulvestrant and was never marketed. It is a silent antagonist of the estrogen receptor (ER) with no intrinsic estrogenic activity and hence is a pure antiestrogen, unlike selective estrogen receptor modulators (SERMs) like tamoxifen. The drug was under development by AstraZeneca for the treatment of breast cancer but was discontinued in favor of fulvestrant, which is very similar to ICI-164384 but is more potent in comparison. See also * Cytestrol acetate * Ethamoxytriphetol Ethamoxytriphetol (developmental code name MER-25) is a synthetic nonsteroidal antiestrogen that was studied clinically in the late 1950s and early 1960s but was never marketed. MER-25 was first reported in 1958, and was the first antiestrogen t ... * TAS-108 References External links ICI- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TAS-108
TAS-108, also known as SR-16234, is a drug discovered by Masato Tanabe and under development by SRI International and Taiho Pharmaceutical. It is a steroid hormone that has shown signs of treating and preventing breast cancer, even in patients where tamoxifen has failed. Development Masato Tanabe's team at SRI has focused on the development of steroid hormones. A compound discovered in a previous SRI contract from the National Institutes of Health showed potential – it acted like "anti-estrogen" in the breasts and uterus but like normal estrogen elsewhere in the body, and was more "tissue-selective". A contract was proposed to Taiho Pharmaceutical in July 1996, and within six years and slightly under $3 million (an unusually short amount of time), two new drugs were discovered and tested on people (particularly people for which tamoxifen has failed): SR-16234 and SR-16287. The first of those, SR-16234, also inhibited the growth of blood vessels angiogenesis and accelerated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen Receptor
Estrogen receptors (ERs) are proteins found in cell (biology), cells that function as receptor (biochemistry), receptors for the hormone estrogen (17β-estradiol). There are two main classes of ERs. The first includes the intracellular estrogen receptors, namely ERα and ERβ, which belong to the nuclear receptor family. The second class consists of membrane estrogen receptors (mERs), such as GPER (GPR30), ER-X, and Gq-mER, Gq-mER, which are primarily G protein-coupled receptors. This article focuses on the nuclear estrogen receptors (ERα and ERβ). Upon activation by estrogen, intracellular ERs undergo protein targeting, translocation to the nucleus where they bind to specific DNA sequences. As DNA-binding transcription factors, they regulate the activity of various genes. However, ERs also exhibit functions that are independent of their DNA-binding capacity. These non-genomic actions contribute to the diverse effects of estrogen signaling in cells. Estrogen receptors (ERs) b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethamoxytriphetol
Ethamoxytriphetol (developmental code name MER-25) is a synthetic nonsteroidal antiestrogen that was studied clinically in the late 1950s and early 1960s but was never marketed. MER-25 was first reported in 1958, and was the first antiestrogen to be discovered. It has been described as "essentially devoid of estrogenic activity" and as having "very low estrogenic activity in all species tested". However, some estrogenic effects in the uterus have been observed, so it is not a pure antiestrogen (that is, a silent antagonist of the estrogen receptor (ER)) but is, instead, technically a selective estrogen receptor modulator (SERM). For all intents and purposes, it is a nearly pure antiestrogen, however. MER-25 produces antifertility effects in animals, and garnered interest as a potential hormonal contraceptive. However, clinical development was discontinued due to its low potency and the incidence of unacceptable central nervous system side effects, including hallucinations and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytestrol Acetate
Cytestrol acetate is a steroidal antiestrogen and a cytostatic antineoplastic agent (i.e., chemotherapeutic) which was developed for the treatment of breast cancer but was never marketed. It is an 11α-hydroxylated derivative of ethinylestradiol in which a bis(2-chloroethyl)amine nitrogen mustard moiety has been attached as an ester at the C3 position and acetate esters have been attached at the C11α and C17β positions. The mechanism of action of cytestrol acetate in breast cancer is two-fold: (1) acting as an antiestrogen similarly to fulvestrant or ICI-164384; and (2) having cytostatic actions via the carbamate–nitrogen mustard moiety analogously to estramustine phosphate. The drug shows potent efficacy against breast cancer superior to that of tamoxifen in ''in vitro'' models. See also * List of hormonal cytostatic antineoplastic agents * List of Russian drugs This page is a list of Russian drugs, or drugs that were developed in Russia, the former Soviet Union, and/ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
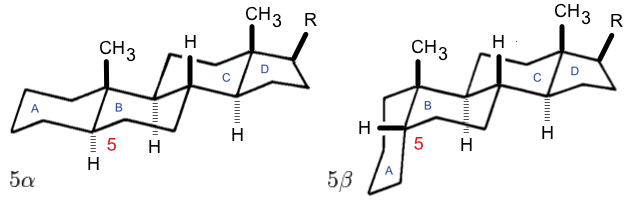
Steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signal transduction, signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in Fungus, fungi, plants, and animals. All steroids are manufactured in cells from a sterols, sterol: Cholesterol, cholesterol (animals), lanosterol (opisthokonts), or cycloartenol (plants). All three of these molecules are produced via Cyclic compound, cyclization of the triterpene squalene. Structure The steroid nucleus (parent structure, core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breast Cancer
Breast cancer is a cancer that develops from breast tissue. Signs of breast cancer may include a Breast lump, lump in the breast, a change in breast shape, dimpling of the skin, Milk-rejection sign, milk rejection, fluid coming from the nipple, a newly inverted nipple, or a red or scaly patch of skin. In those with Metastatic breast cancer, distant spread of the disease, there may be bone pain, swollen lymph nodes, shortness of breath, or yellow skin. Risk factors for developing breast cancer include obesity, a Sedentary lifestyle, lack of physical exercise, alcohol consumption, hormone replacement therapy during menopause, ionizing radiation, an early age at Menarche, first menstruation, having children late in life (or not at all), older age, having a prior history of breast cancer, and a family history of breast cancer. About five to ten percent of cases are the result of an inherited genetic predisposition, including BRCA mutation, ''BRCA'' mutations among others. Breast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diols
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drugs Developed By AstraZeneca
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via inhalation, injection, smoking, ingestion, absorption via a patch on the skin, suppository, or dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to treat, cure, prevent, or diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used for a limited duration, or on a regular basis for chronic disorders. Classification Pharmaceutical drugs are often classified into drug classes—groups of related drugs that have simila ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiestrogens
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inhibiting or suppressing estrogen production., Antiestrogens are one of three types of sex hormone antagonists, the others being antiandrogens and antiprogestogens. Antiestrogens are commonly used to stop steroid hormones, estrogen, from binding to the estrogen receptors leading to the decrease of estrogen levels. Decreased levels of estrogen can lead to complications in sexual development. Types and examples Antiestrogens include selective estrogen receptor modulators (SERMs) like tamoxifen, clomifene, and raloxifene, the ER silent antagonist and selective estrogen receptor degrader (SERD) fulvestrant, aromatase inhibitors (AIs) like anastrozole, and antigonadotropins including androgens/anabolic steroids, progestogens, and GnRH ana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tamoxifen
Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome. Tamoxifen is typically taken daily by mouth for five years for breast cancer. Serious side effects include a small increased risk of uterine cancer, stroke, vision problems, and pulmonary embolism. Common side effects include irregular periods, weight loss, and hot flashes. It may cause harm to the baby if taken during pregnancy or breastfeeding. It is a selective estrogen-receptor modulator (SERM) and works by decreasing the growth of breast cancer cells. It is a member of the triphenylethylene group of compounds. Tamoxifen was initially made in 1962, by chemist Dora Richardson. It is on the World Health Organization's List of Essential Medicines. Tamoxifen is available as a generic medication. In 2020, it was the 317th mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AstraZeneca
AstraZeneca plc () (AZ) is a British-Swedish multinational pharmaceutical and biotechnology company with its headquarters at the Cambridge Biomedical Campus in Cambridge, UK. It has a portfolio of products for major diseases in areas including oncology, cardiovascular, gastrointestinal, infection, neuroscience, respiratory, and inflammation. The company was founded in 1999 through the merger of the Swedish Astra AB and the British Zeneca Group (itself formed by the demerger of the pharmaceutical operations of Imperial Chemical Industries in 1993). Its portfolio includes primary and speciality care, coverage for rare diseases, and a robust global presence across various regions. Since the merger it has been among the world's largest pharmaceutical companies and has made numerous corporate acquisitions, including Cambridge Antibody Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) and Definiens (by MedImmune in 2014). It has its research and development conc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |