|
Hydroxysteroid Dehydrogenase
Hydroxysteroid dehydrogenases (HSDs) are a group of alcohol oxidoreductases that catalyze the dehydrogenation of hydroxysteroids. These enzymes also catalyze the reverse reaction, acting as ketosteroid reductases (KSRs). There are four types, classified by the number of the position acted upon: See also * Steroidogenic enzyme * Steroid hydroxylase A steroid hydroxylase is a class of hydroxylase enzymes involved in the biosynthesis of steroids. See also * Steroidogenic enzyme Additional images File:Steroidogenesis.svg, Steroidogenesis Image:Steroid numbering.svg, Steroid nomenclature � ... External links * {{Portal bar, Biology, border=no EC 1.1.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
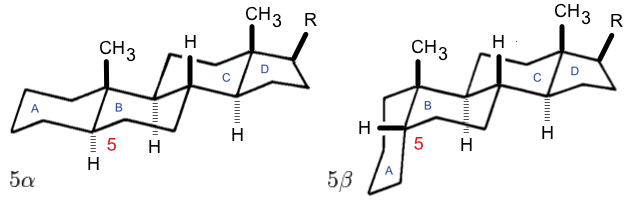
Steroidogenesis
A steroid is an organic compound with four fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in fungi, plants, and animals. All steroids are manufactured in cells from a sterol: cholesterol (animals), lanosterol ( opisthokonts), or cycloartenol (plants). All three of these molecules are produced via cyclization of the triterpene squalene. Structure The steroid nucleus ( core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in four fused rings: three six-member cyclohexane rings (rings A, B and C in the first illustrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Androstenedione
Androstenedione, or 4-androstenedione (abbreviated as A4 or Δ4-dione), also known as androst-4-ene-3,17-dione, is an endogenous weak androgen steroid hormone and intermediate in the biosynthesis of estrone and of testosterone from dehydroepiandrosterone (DHEA). It is closely related to androstenediol (androst-5-ene-3β,17β-diol). Function Androstenedione is a precursor of testosterone and other androgens, as well as of estrogens like estrone, in the body. In addition to functioning as an endogenous prohormone, androstenedione also has weak androgenic activity in its own right. Androstenedione has been found to possess some estrogenic activity, similarly to other DHEA metabolites. However, in contrast to androstenediol, its affinity for the estrogen receptors is very low, with less than 0.01% of the affinity of estradiol for both the ERα and ERβ. Adrenarche In children aged 6 to 8 years old, there is a rise in androstenedione secretion along with DHEA during adre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroidogenic Enzyme
__NOTOC__ Steroidogenic enzymes are enzymes that are involved in steroidogenesis and steroid biosynthesis. They are responsible for the biosynthesis of the steroid hormones, including sex steroids (androgens, estrogens, and progestogens) and corticosteroids (glucocorticoids and mineralocorticoids), as well as neurosteroids, from cholesterol. Steroidogenic enzymes are most highly expressed in classical steroidogenic tissues, such as the testis, ovary, and adrenal cortex, but are also present in other tissues in the body. List of steroidogenic enzymes * Steroid desmolases ** Cholesterol side-chain cleavage enzyme (20,22-desmolase) – steroid synthesis ** 17,20-Lyase (17,20-desmolase) – androgen synthesis * Steroid hydroxylases ** 11β-Hydroxylase – corticosteroid synthesis ** 17α-Hydroxylase – androgen and glucocorticoid synthesis ** 18-Hydroxylase (aldosterone synthase) – mineralocorticoid synthesis ** 21-Hydroxylase – corticosteroid synthesis ** Cytochrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
20α-Dihydroprogesterone
20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen. It is a metabolite of progesterone, formed by the 20α-hydroxysteroid dehydrogenases (20α-HSDs) AKR1C1, AKR1C2, and AKR1C3 and the 17β-hydroxysteroid dehydrogenase (17β-HSD) HSD17B1. 20α-DHP can be transformed back into progesterone by 20α-HSDs and by the 17β-HSD HSD17B2. HSD17B2 is expressed in the human endometrium and cervix among other tissues. In animal studies, 20α-DHP has been found to be selectively taken up into and retained in target tissues such as the uterus, brain, and skeletal muscle. 20α-DHP has very low affinity for the progesterone receptor and is much less potent as a progestogen in comparison to progesterone, with about one-fifth of the relative progestogenic activity. It has also been found to act as an aromatase inhibitor and to inhibit the production of estrogen in breast tissue ''in vitro''. A single 200-mg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
20β-Hydroxysteroid Dehydrogenase
In enzymology, a 3alpha(or 20beta)-hydroxysteroid dehydrogenase () is an enzyme that catalyzes the chemical reaction : androstan-3alpha,17beta-diol + NAD+ \rightleftharpoons 17beta-hydroxyandrostan-3-one + NADH + H+ Thus, the two substrates of this enzyme are androstan-3alpha,17beta-diol and NAD+, whereas its 3 products are 17beta-hydroxyandrostan-3-one, NADH, and H+. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is 3alpha(or 20beta)-hydroxysteroid:NAD+ oxidoreductase. Other names in common use include cortisone reductase, (R)-20-hydroxysteroid dehydrogenase, dehydrogenase, 20beta-hydroxy steroid, Delta4-3-ketosteroid hydrogenase, 20beta-hydroxysteroid dehydrogenase, 3alpha,20beta-hydroxysteroid:NAD+-oxidoreductase, NADH-20beta-hydroxysteroid dehydrogenase, and 20beta-HSD. This enzyme participates in bile acid biosynthesis and c21-steroid ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estradiol
Estradiol (E2), also called oestrogen, oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of female reproductive cycles such as estrous and menstrual cycles. Estradiol is responsible for the development of female secondary sexual characteristics such as the breasts, widening of the hips and a female pattern of fat distribution. It is also important in the development and maintenance of female reproductive tissues such as the mammary glands, uterus and vagina during puberty, adulthood and pregnancy. It also has important effects in many other tissues including bone, fat, skin, liver, and the brain. Though estradiol levels in males are much lower than in females, estradiol has important roles in males as well. Apart from humans and other mammals, estradiol is also found in most vertebrates and crustaceans, insects, fish, and other animal species. Estradiol is produced within the follicles of the ovaries and in oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrone
Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue. Relative to estradiol, both estrone and estriol have far weaker activity as estrogens. Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol. It is both a precursor and metabolite of estradiol. In addition to its role as a natural hormone, estrone has been used as a medication, for instance in menopausal hormone therapy; for information on estrone as a medication, see the estrone (medication) article. Biological activity Estrone is an estrogen, specifically an agonist of the estrogen receptors ERα and ERβ. It is a far less potent estrogen than is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Androstanedione
Androstanedione, also known as 5α-androstanedione or as 5α-androstane-3,17-dione, is a naturally occurring androstane (5α-androstane) steroid and an endogenous metabolite of androgens like testosterone, dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), and androstenedione. It is the C5 epimer of etiocholanedione (5β-androstanedione). Androstanedione is formed from androstenedione by 5α-reductase and from DHT by 17β-hydroxysteroid dehydrogenase. It has some androgen An androgen (from Greek ''andr-'', the stem of the word meaning ) is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This includes ...ic activity. In female genital skin, the conversion of androstenedione into DHT through 5α-androstanedione appears to be more important than the direct conversion of testosterone into DHT. References External links Androstanedione (HMDB0000899) - Human ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
17β-Hydroxysteroid Dehydrogenase
A hydroxysteroid is a molecule derived from a steroid with a hydrogen replaced with a hydroxy group. When the hydroxy group is specifically at the C3 position, hydroxysteroids are referred to as sterols, with an example being cholesterol Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils. Cholesterol is biosynthesis, biosynthesized by all anima .... See also * Hydroxysteroid dehydrogenase * Ketosteroid External links * Alcohols Steroids {{steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cortisone
Cortisone is a pregnene (21-carbon) steroid hormone. It is a naturally-occurring corticosteroid metabolite that is also used as a pharmaceutical prodrug. Cortisol is converted by the action of the enzyme corticosteroid 11-beta-dehydrogenase isozyme 2 into the inactive metabolite cortisone, particularly in the kidneys. This is done by oxidizing the alcohol group at carbon 11 (in the six-membered ring fused to the five-membered ring). Cortisone is converted back to the active steroid cortisol by stereospecific hydrogenation at carbon 11 by the enzyme 11β-Hydroxysteroid dehydrogenase type 1, particularly in the liver. The term "cortisone" is frequently misused to mean either any corticosteroid or hydrocortisone, which is in fact cortisol. Many who speak of receiving a "cortisone shot" or taking "cortisone" are more likely receiving hydrocortisone or one of many other, much more potent synthetic corticosteroids. Cortisone can be administered as a prodrug, meaning it has to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cortisol
Cortisol is a steroid hormone in the glucocorticoid class of hormones and a stress hormone. When used as medication, it is known as hydrocortisone. Cortisol is produced in many animals, mainly by the ''zona fasciculata'' of the adrenal cortex in an adrenal gland. In other tissues, it is produced in lower quantities. By a Circadian rhythm, diurnal cycle, cortisol is released and increases in response to Stress (biology), stress and a low Blood sugar, blood-glucose concentration. It functions to increase blood sugar through gluconeogenesis, suppress the immune system, and aid in the metabolism of calories. It also decreases bone formation. These stated functions are carried out by cortisol binding to glucocorticoid or mineralocorticoid receptors inside a cell, which then bind to DNA to affect gene expression. Health effects Metabolic response Metabolism of glucose Cortisol plays a crucial role in regulating glucose metabolism and promotes gluconeogenesis (glucose synthes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |