|
Heterodiamond
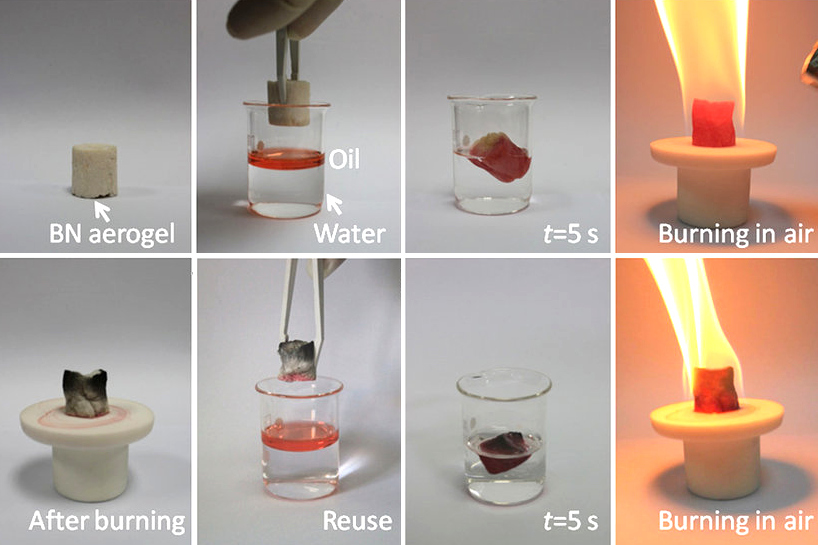
Heterodiamond is a superhard material containing boron, carbon, and nitrogen (BCN). It is formed at high temperatures and high pressures, e.g., by application of an explosive shock wave to a mixture of diamond and cubic boron nitride (c-BN). The heterodiamond is a polycrystalline material coagulated with nano-crystallites and the fine powder is tinged with deep bluish black. The heterodiamond has both the high hardness of diamond and the excellent heat resistance of cubic BN. These characteristic properties are due to the diamond structure combined with the sp3 σ-bonds among carbon and the heteroatoms. Cubic BC2N can be synthesized from graphite-like BC2N at pressures above 18 GPa and temperatures higher than . The bulk modulus of c-BC2N is 282 GPa which is one of the highest bulk moduli known for any solid, and is exceeded only by the bulk moduli of diamond and c-BN. The hardness of c-BC2N is higher than that of c-BN single crystals. References See also * Boron nitride ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula B N. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. History Boron nitride was discovered by chemistry teacher of the Liverpool Institute in 1842 via reduction of boric acid with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sp3 Bond
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Compounds
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides. Halides The trihalides adopt a planar trigonal structure. These compounds are Lewis acids in that they readily form adducts with electron-pair donors, which are called Lewis bases. For example, fluoride (F−) and boron trifluoride (BF3) combined to give the tetrafluoroborate anion, BF4−. Boron trifluoride is used in the petrochemical industry as a catalyst. The halides react with water to form boric acid. Oxygen compounds Boron is found in nature on Earth almost entirely as various oxides of B(III), often associated with other elements. More than one hundred borate minerals contain boron in oxidation state +3. These minerals resemble silicates in some respect, although boron is often found not only in a tetrahedral coordination with oxygen, but also in a trigonal planar configuration. Unlik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superhard Materials
A superhard material is a material with a hardness value exceeding 40 gigapascals ( GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings. Diamond is the hardest known material to date, with a Vickers hardness in the range of 70–150 GPa. Diamond demonstrates both high thermal conductivity and electrically insulating properties, and much attention has been put into finding practical applications of this material. However, diamond has several limitations for mass industrial application, including its high cost and oxidation at temperatures above 800 °C. In addition, diamond dissolves in iron and forms iron carbides at high temperatures and therefore is ineffic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bulk Modulus
The bulk modulus (K or B or k) of a substance is a measure of the resistance of a substance to bulk compression. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume. Other moduli describe the material's response ( strain) to other kinds of stress: the shear modulus describes the response to shear stress, and Young's modulus describes the response to normal (lengthwise stretching) stress. For a fluid, only the bulk modulus is meaningful. For a complex anisotropic solid such as wood or paper, these three moduli do not contain enough information to describe its behaviour, and one must use the full generalized Hooke's law. The reciprocal of the bulk modulus at fixed temperature is called the isothermal compressibility. Definition The bulk modulus K (which is usually positive) can be formally defined by the equation :K=-V\frac , where P is pressure, V is the initial volume of the substance, and dP/dV deno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
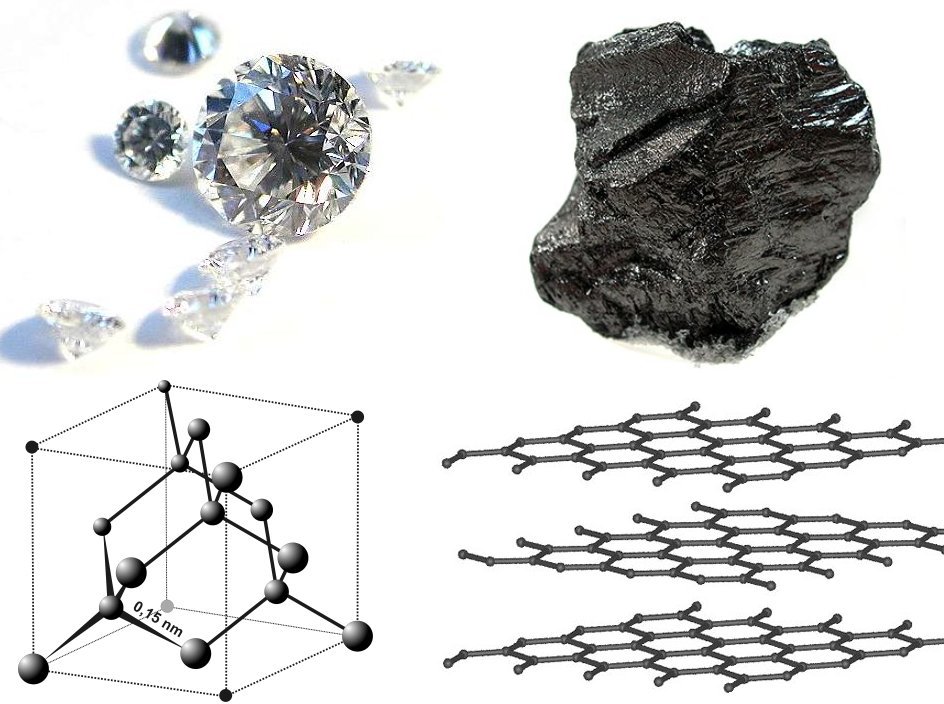
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation under certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the '' reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that could be produced based ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen. Organic chemistry In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular structure. Typical heteroatoms are nitrogen (N), oxygen (O), sulfur (S), phosphorus (P), chlorine (Cl), bromine (Br), and iodine (I), as well as the metals lithium (Li) and magnesium (Mg). Proteins It can also be used with highly specific meanings in specialised contexts. In the description of protein structure, in particular in the Protein Data Bank file format, a heteroatom record (HETATM) describes an atom as belonging to a small molecule cofactor rather than being part of a biopolymer chain. Zeolites In the context of zeolites, the term ''heteroatom'' refers to partial isomorphous substitution of the typical framework atoms (silicon, aluminium, and phosphorus) by other elements such as beryllium, vanadium, and chromium. The goal i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superhard Material
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high Covalent bond, bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and Cutting tool (machining), cutting tools, disc brakes, and wear-resistant and protective coatings. Diamond is the hardest known material to date, with a Vickers hardness in the range of 70–150 GPa. Diamond demonstrates both high thermal conductivity and Insulator (electrical), electrically insulating properties, and much attention has been put into finding practical applications of this material. However, diamond has several limitations for mass industrial application, including its high cost and oxidation at temperatures above 800 °C. In addition, diamond dissolves in iron and forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond Cubic
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, including α-tin, the semiconductors silicon and germanium, and silicon–germanium alloys in any proportion. There are also crystals, such as the high-temperature form of cristobalite, which have a similar structure, with one kind of atom (such as silicon in cristobalite) at the positions of carbon atoms in diamond but with another kind of atom (such as oxygen) halfway between those (see :Minerals in space group 227). Although often called the diamond lattice, this structure is not a lattice in the technical sense of this word used in mathematics. Crystallographic structure Diamond's cubic structure is in the Fdm space group (space group 227), which follows the face-centered cubic Bravais lattice. The lattice describes the repeat pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |