|
Hemidesmus Indicus
''Hemidesmus indicus'', Indian sarsaparilla, is a species of plant found in South Asia. It occurs over the greater part of India, from the upper Gangetic plain eastwards to Assam and in some places in central, western and South India. The root is a substitute for sarsaparilla (the dried root of the tropical species of ''Smilax'', Smilacaceae; in India ''Smilax aspera'' L., and ''Smilax ovalifolia'' Roxb.). It should be distinguished from Mexican Sarsaparilla ''Smilax'' ''aristolochiifolia'' Mill. and Jamaican Sarsaparilla ''Smilax ornata'' Hook.f.. Names In India, it is called ''ananthamoola'', also known locally in Southern India as ''naruneendi'' or ''nannari''. Description It is a slender, twining, sometimes prostrate or semi-erect climber. Roots are woody and aromatic. The stem is numerous, slender, terete, thickened at the nodes. The leaves are opposite, short-petioled, very variable, elliptic-oblong to linear-lanceolate. The flowers are greenish outside, purplish ins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Linnaeus
Carl Linnaeus (23 May 1707 – 10 January 1778), also known after ennoblement in 1761 as Carl von Linné,#Blunt, Blunt (2004), p. 171. was a Swedish biologist and physician who formalised binomial nomenclature, the modern system of naming organisms. He is known as the "father of modern Taxonomy (biology), taxonomy". Many of his writings were in Latin; his name is rendered in Latin as and, after his 1761 ennoblement, as . Linnaeus was the son of a curate and was born in Råshult, in the countryside of Småland, southern Sweden. He received most of his higher education at Uppsala University and began giving lectures in botany there in 1730. He lived abroad between 1735 and 1738, where he studied and also published the first edition of his ' in the Netherlands. He then returned to Sweden where he became professor of medicine and botany at Uppsala. In the 1740s, he was sent on several journeys through Sweden to find and classify plants and animals. In the 1750s and 1760s, he co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
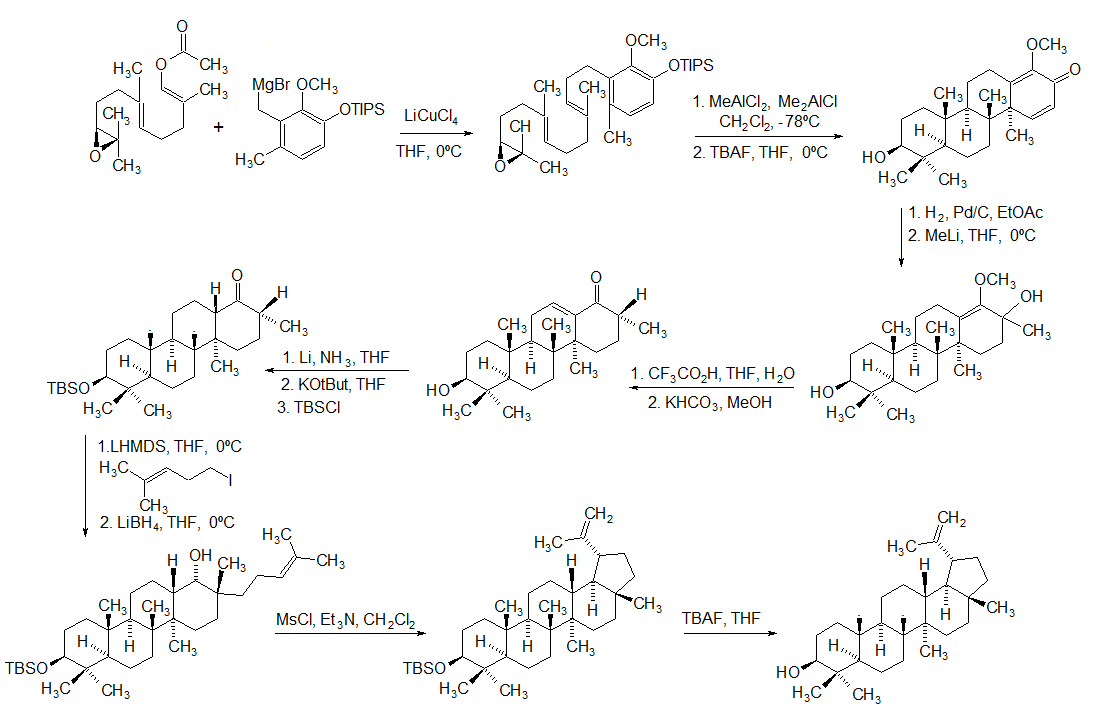
Lupeol
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity. Natural occurrences Lupeol is found in a variety of plants, including mango, '' Acacia visco'' and '' Abronia villosa''. It is also found in dandelion coffee. Lupeol is present as a major component in ''Camellia japonica'' leaf. Total synthesis The first total synthesis of lupeol was reported by Gilbert Stork ''et al''. In 2009, Surendra and Corey reported a more efficient and enantioselective total synthesis of lupeol, starting from (''1E,5E'')-8- ''2S'')-3,3-dimethyloxiran-2-yl2,6-dimethylocta-1,5-dienyl acetate by use of a polycyclization. Biosynthesis Lupeol is produced by several organisms from squalene epoxide. Dammarane and baccharane skeletons are formed as intermediates. The reactions are catalyzed by the enzyme lupeol synthase. A recent study on the metabolomics of ''Camellia japonica'' leaf revealed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucoderma
Vitiligo (, ) is a chronic autoimmune disorder that causes patches of skin to lose pigment or color. The cause of vitiligo is unknown, but it may be related to immune system changes, genetic factors, stress, or sun exposure, and susceptibility to it may be affected by regional environmental risk factors, especially early in life. Treatment options include topical medications, light therapy, surgery and cosmetics. The condition causes patches of a light peachy color of any size, which can appear on any place on the body; in particular, nonsegmental vitiligo, the common form, tends to progress, affecting more of the skin over time. Vitiligo spots on the skin can also vary in pigmentation over long periods, although they will stay in relatively the same areas. Signs and symptoms The only sign of vitiligo is the presence of pale patchy areas of depigmented skin which tend to occur on the extremities. Some people may experience itching before a new patch appears. The patches ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rutin
Rutin (rutoside, quercetin-3-O-rutinoside or sophorin) is the glycoside combining the flavonol quercetin and the disaccharide rutinose (α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranose). It is a flavonoid glycoside found in a wide variety of plants, including citrus. Occurrences Rutin is one of the phenolic compounds found in the plant species ''Carpobrotus edulis''. Its name comes from the name of '' Ruta graveolens'', a plant that also contains rutin. Various citrus fruit peels contain 32 to 49 mg/g of flavonoids expressed as rutin equivalents. Citrus leaves contain rutin at concentrations of 11 and 7 g/kg in orange and lime trees, respectively. In 2021, Samoan researchers identified rutin in the native plant ''matalafi'' ('' Psychotria insularum''). Metabolism The enzyme quercitrinase found in ''Aspergillus flavus'' is in the rutin catabolic pathway. In food Rutin is a citrus flavonoid glycoside found in many plants, including buckwheat, the leaves and petioles of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperoside
Hyperoside is a chemical compound. It is the 3-''O''-galactoside of quercetin. Natural occurrences Hyperoside has been isolated from ''Drosera rotundifolia'', from the Lamiaceae ''Stachys sp.'' and ''Prunella vulgaris'', from ''Rumex acetosella'', '' Cuscuta chinensis'' seeds, from St John's wort and from ''Camptotheca acuminata''. It is one of the phenolic compounds in the invasive plant ''Carpobrotus edulis'' and contributes to the antibacterial properties of the plant. In '' Rheum nobile'' and '' R. rhaponticum'', it serves as a UV blocker found in the bracts. It is also found in ''Geranium niveum ''Geranium niveum'' is a plant species in the genus ''Geranium''. It is a medicinal herb widely used by the Tarahumara Indians of Mexico. Geranin A Geranin A is an A type proanthocyanidin of the propelargonidin sub type. Its structure is ep ...'' and '' Taxillus kaempferi''.The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoid
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a Heterocyclic compound, heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenylchromone, chromen-4-one (3-phenyl-1,4-benzopyran, benzopyrone) structure *neoflavonoids, derived from 4-phenylcoumarin (4-phenyl-1,2-benzopyran, benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins (flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tannin
Tannins (or tannoids) are a class of astringent, polyphenolic biomolecules that bind to and Precipitation (chemistry), precipitate proteins and various other organic compounds including amino acids and alkaloids. The term ''tannin'' is widely applied to any large polyphenolic compound containing sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with various macromolecules. The term ''tannin'' (from scientific French ''tannin'', from French ''tan'' "crushed oak bark", ''tanner'' "to tan", cognate with English language, English ''tanning'', Medieval Latin ''tannare'', from Proto-Celtic ''*tannos'' "oak") refers to the abundance of these compounds in oak Bark (botany), bark, which was used in Tanning (leather), tanning animal Hide (skin), hides into leather. The tannin compounds are widely distributed in many species of plants, where they play a role in protection from predation (acting as pesticides) and might help in regulating plant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpene
Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' often are used i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminium'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic anion , or . Most of the approximately 5 million tonnes of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common building block for biosynthesis. Nomenclature and common formula When part of a salt, the formula of the acetate i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |