|
Hahnium
Dubnium is a Synthetic element, synthetic chemical element with the Symbol (chemistry), symbol Db and atomic number 105. It is highly radioactive: the most stable known isotopes of dubnium, isotope, dubnium-268, has a half-life of about 16 hours. This greatly limits extended research on the element. Dubnium does not occur naturally on Earth and is produced artificially. The Soviet Joint Institute for Nuclear Research (JINR) claimed the first discovery of the element in 1968, followed by the American Lawrence Berkeley Laboratory in 1970. Both teams proposed their names for the new element and used them without formal approval. The long-standing dispute was resolved in 1993 by an official investigation of the discovery claims by the Transfermium Working Group, formed by the International Union of Pure and Applied Chemistry and the International Union of Pure and Applied Physics, resulting in credit for the discovery being officially shared between both teams. The element was for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Symbol (chemistry)
Chemical symbols are the abbreviations used in chemistry for chemical elements, functional groups and chemical compounds. Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek vocabulary. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a new Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter temporary symbol may be assigned to a newly synthesized (or not yet synthesized) element. For example, "Uno" was the temporary symbol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transfermium Wars
The names for the chemical elements 104 to 106 were the subject of a major controversy starting in the 1960s, described by some nuclear chemists as the Transfermium Wars because it concerned the elements following fermium (element 100) on the periodic table. This controversy arose from disputes between American scientists and Soviet scientists as to which had first isolated these elements. The final resolution of this controversy in 1997 also decided the names of elements 107 to 109. Controversy By convention, naming rights for newly discovered chemical elements go to their discoverers. For elements 104, 105, and 106, there was a controversy between Soviet researchers at the Joint Institute for Nuclear Research and American researchers at Lawrence Berkeley National Laboratory regarding which group had discovered them first. Both parties suggested their own names for elements 104 and 105, not recognizing the other's name. The American name of seaborgium for element 106 was also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
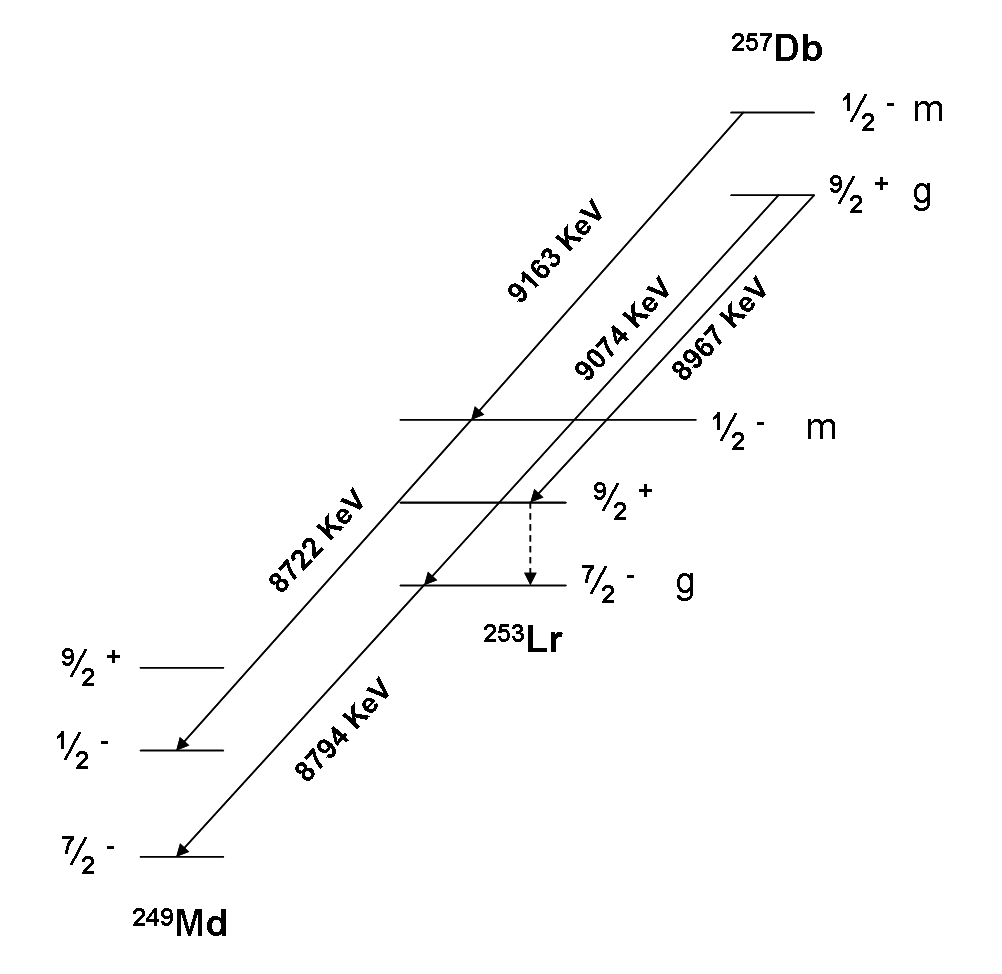
Isotopes Of Dubnium
Dubnium (105Db) is a synthetic element, thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 261Db in 1968. The 13 known radioisotopes are from 255Db to 270Db, and 1–3 isomers. The longest-lived known isotope is 268Db with a half-life of 16 hours. List of isotopes , - , rowspan=2, 255Db , rowspan=2 style="text-align:right" , 105 , rowspan=2 style="text-align:right" , 150 , rowspan=2, 255.10707(45)# , rowspan=2, , α (~50%) , 251Lr , rowspan=2, , - , SF (~50%) , (various) , - , rowspan=3, 256Db , rowspan=3 style="text-align:right" , 105 , rowspan=3 style="text-align:right" , 151 , rowspan=3, 256.10789(26)# , rowspan=3, 1.9(4) s[] , α (~64%) , 252Lr , rowspan=3, , - , SF (~0.02%) , (various) , - , beta decay, β+ (~36%) , 256Rf , - , rowspan=3, 257Db , rowspan=3 style="text-align:right" , 105 , rowspan=3 style="text-align:right" , 152 , rowspan=3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; thus, they are called "synthetic", "artificial", or "man-made". The synthetic elements are those with atomic numbers 95–118, as shown in purple on the accompanying periodic table: these 24 elements were first created between 1944 and 2010. The mechanism for the creation of a synthetic element is to force additional protons into the nucleus of an element with an atomic number lower than 95. All synthetic elements are unstable, but they decay at widely varying rates: the half-lives of their longest-lived isotopes range from microseconds to millions of years. Five more elements that were created artificially are strictly speaking not ''synthetic'' because they were later found in nature in trace quantities: 43Tc, 61Pm, 85At, 93Np, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable; the half-lives of its naturally occurring isotopes range between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite. In nature, uranium is found as uranium-238 (99.2739 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of and a mass of . For example, uranium-238 decays to form thorium-234. While alpha particles have a charge , this is not usually shown because a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms. Alpha decay typically occurs in the heaviest nuclides. Theoretically, it can occur only in nuclei somewhat heavier than nickel (element 28), where the overall binding energy per nucleon is no longer a maximum and the nuclides are therefore unstable toward sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neon-22
Neon (10Ne) possesses three stable isotopes: , , and . In addition, 17 radioactive isotopes have been discovered, ranging from to , all short-lived. The longest-lived is with a half-life of . All others are under a minute, most under a second. The least stable is with a half-life of (). See isotopes of carbon for notes about the measurement. Light radioactive neon isotopes usually decay to fluorine or oxygen, while heavier ones decay to sodium. List of isotopes , - , , style="text-align:right" , 10 , style="text-align:right" , 5 , , [] , proton emission, 2p , , (3/2−) , , , - , , style="text-align:right" , 10 , style="text-align:right" , 6 , , > [ , - , , style="text-align:right" , 10 , style="text-align:right" , 11 , , colspan=3 align=center, Stable , 3/2+ , , ref name="Isotopic Composition of Elements" /> , - , , style="text-align:right" , 10 , style="text-align:right" , 12 , , colspan=3 align=center, Stable , 0+ , , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Americium-243
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95 (the number of protons in the nucleus of the americium atom). Despite being an order of magnitude longer lived than , the former is harder to obtain than the latter as more of it is present in spent nuclear fuel. Nineteen radioisotopes of americium—223Am, 229Am, 230Am, and those ranging from 232Am to 247Am—have been characterized, with the most stable being 243Am with a half-life of 7,370 years, and 241Am with a half-life of 432.2 years. All of the remaining radioactive isotopes have half-lives that are less than 51 hours, and the majority of these have half-lives that are less than 100 minutes. This element also has 8 meta states, with the most stable being 242m1Am (t1/2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soviet Union
The Soviet Union,. officially the Union of Soviet Socialist Republics. (USSR),. was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 to 1991. A flagship communist state, it was nominally a Federation, federal union of Republics of the Soviet Union, fifteen national republics; in practice, both Government of the Soviet Union, its government and Economy of the Soviet Union, its economy were highly Soviet-type economic planning, centralized until its final years. It was a one-party state governed by the Communist Party of the Soviet Union, with the city of Moscow serving as its capital as well as that of its largest and most populous republic: the Russian Soviet Federative Socialist Republic, Russian SFSR. Other major cities included Saint Petersburg, Leningrad (Russian SFSR), Kyiv, Kiev (Ukrainian Soviet Socialist Republic, Ukrainian SSR), Minsk (Byelorussian Soviet Socialist Republic, Byelorussian SSR), Tas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moscow Oblast
Moscow Oblast ( rus, Моско́вская о́бласть, r=Moskovskaya oblast', p=mɐˈskofskəjə ˈobləsʲtʲ), or Podmoskovye ( rus, Подмоско́вье, p=pədmɐˈskovʲjə, literally " under Moscow"), is a federal subject of Russia (an oblast). With a population of 7,095,120 ( 2010 Census) living in an area of , it is one of the most densely populated regions in the country and is the second most populous federal subject. The oblast has no official administrative center; its public authorities are located in Moscow and Krasnogorsk (Moscow Oblast Duma and government), and also across other locations in the oblast.According to Article 24 of the Charter of Moscow Oblast, the government bodies of the oblast are located in the city of Moscow and throughout the territory of Moscow Oblast. However, Moscow is not named the official administrative center of the oblast. Located in European Russia between latitudes 54° and 57° N and longitudes 35° and 41° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Discovery Of The Chemical Elements
The discovery of the 118 chemical elements known to exist as of 2022 is presented in chronological order. The elements are listed generally in the order in which each was first defined as the pure element, as the exact date of discovery of most elements cannot be accurately determined. There are plans to synthesize more elements, and it is not known how many elements are possible. Each element's name, atomic number, year of first report, name of the discoverer, and notes related to the discovery are listed. Periodic table of elements Ancient discoveries Modern discoveries Graphics See also * History of the periodic table * Periodic table * Extended periodic table * ''The Mystery of Matter: Search for the Elements'' (2014/2015 PBS film) * Transfermium Wars References External linksHistory of the Origin of the Chemical Elements and Their DiscoverersLast updated by Boris Pritychenko on March 30, 2004 [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transactinide Chemistry Apparatus Dubna
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the last actinide is lawrencium (atomic number 103). By definition, superheavy elements are also transuranium elements, i.e., having atomic numbers greater than that of uranium (92). Depending on the definition of group 3 adopted by authors, lawrencium may also be included to complete the 6d series. Glenn T. Seaborg first proposed the actinide concept, which led to the acceptance of the actinide series. He also proposed a transactinide series ranging from element 104 to 121 and a superactinide series approximately spanning elements 122 to 153 (although more recent work suggests the end of the superactinide series to occur at element 157 instead). The transactinide seaborgium was named in his honor. Superheavy elements are radioactive and have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |