|
Hafnium(IV) Silicate
Hafnium silicate is the hafnium(IV) salt of silicic acid with the chemical formula of HfSiO4. Thin films of hafnium silicate and zirconium silicate grown by atomic layer deposition, chemical vapor deposition or MOCVD, can be used as a high-k dielectric as a replacement for silicon dioxide in modern semiconductor devices. The addition of silicon to hafnium oxide increases the band gap, while decreasing the dielectric constant. Furthermore, it increases the crystallization temperature of amorphous films and further increases the material's thermal stability with Si at high temperatures. Nitrogen is sometimes added to hafnium silicate for improving the thermal stability and electrical properties of devices. Natural occurrence Hafnon is the natural form of hafnium orthosilicate. Its name suggests the mineral is the Hf analogue of much more common zircon Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1922, by Dirk Coster and George de Hevesy. Hafnium is named after , the Latin name for Copenhagen, where it was discovered. Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuits at 45 nanometers and smaller feature lengths. Some superalloys used for special applications contain hafnium in combination with niobium, titanium, or tungsten. Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rods in nuclear power plants, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloys used in nuclear r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
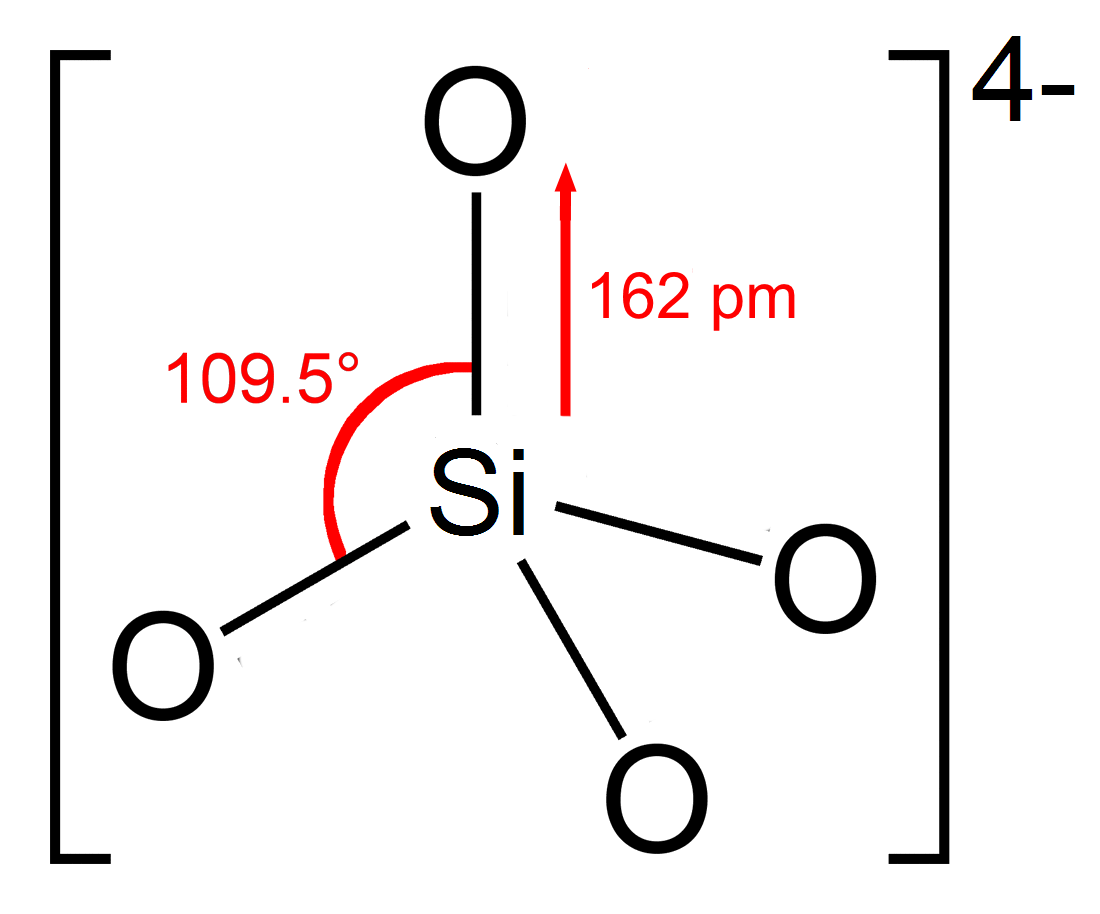
Silicates
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt (chemistry), salt of such anions, such as sodium metasilicate; or any ester containing the corresponding Moiety (chemistry), chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicic acid, hexafluorosilicate . Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In most silicates, a silicon atom occupies th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zircon
Zircon () is a mineral belonging to the group of nesosilicates and is a source of the metal zirconium. Its chemical name is zirconium(IV) silicate, and its corresponding chemical formula is Zr SiO4. An empirical formula showing some of the range of substitution in zircon is (Zr1–y, REEy)(SiO4)1–x(OH)4x–y. Zircon precipitates from silicate melts and has relatively high concentrations of high field strength incompatible elements. For example, hafnium is almost always present in quantities ranging from 1 to 4%. The crystal structure of zircon is tetragonal crystal system. The natural color of zircon varies between colorless, yellow-golden, red, brown, blue, and green. The name derives from the Persian ''zargun'', meaning "gold-hued". This word is changed into " jargoon", a term applied to light-colored zircons. The English word "zircon" is derived from ''Zirkon'', which is the German adaptation of this word. Yellow, orange, and red zircon is also known as " hyacint ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dielectric Constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulator measures the ability of the insulator to store electric energy in an electrical field. Permittivity is a material's property that affects the Coulomb force between two point charges in the material. Relative permittivity is the factor by which the electric field between the charges is decreased relative to vacuum. Likewise, relative permittivity is the ratio of the capacitance of a capacitor using that material as a dielectric, compared with a similar capacitor that has vacuum as its dielectric. Relative permittivity is also commonly known as the dielectric constant, a term still used but deprecated by standards organizations in engineering as well as in chemistry. Definition Relative permittivity is typically denoted as (som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the energy difference (often expressed in electronvolts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote an electron from the valence band to the conduction band. The resulting conduction-band electron (and the electron hole in the valence band) are free to move within the crystal lattice and serve as charge carriers to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium Oxide
Hafnium(IV) oxide is the inorganic compound with the formula . Also known as hafnium dioxide or hafnia, this colourless solid is one of the most common and stable compounds of hafnium. It is an electrical insulator with a band gap of 5.3~5.7 eV. Hafnium dioxide is an intermediate in some processes that give hafnium metal. Hafnium(IV) oxide is quite inert. It reacts with strong acids such as concentrated sulfuric acid and with strong bases. It dissolves slowly in hydrofluoric acid to give fluorohafnate anions. At elevated temperatures, it reacts with chlorine in the presence of graphite or carbon tetrachloride to give hafnium tetrachloride. Structure Hafnia typically adopts the same structure as zirconia (ZrO2). Unlike TiO2, which features six-coordinate Ti in all phases, zirconia and hafnia consist of seven-coordinate metal centres. A variety of other crystalline phases have been experimentally observed, including cubic fluorite (Fmm), tetragonal (P42/nmc), monoclinic (P21/c) a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Silicon is a significant element that is essential for several physiological and metabolic processes in plants. Silicon is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others. These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions ( anions), which results in a compound with no net electric charge (electrically neutral). The constituent ions are held together by electrostatic forces termed ionic bonds. The component ions in a salt can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic, such as ammonium () and carbonate () ions in ammonium carbonate. Salts containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases, such as sodium hydroxide and potassium oxide. Individual ions within a salt usually have multiple near neighbours, so they are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Salts usually form crystalline structures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MOCVD
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures (10 to 760 Torr). As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as light-emitting diodes, its most widespread application. It was first demonstrated in 1967 at North American Aviation (later Rockwell International) Autonetics Division in Anaheim CA by Harold M. Manasevit. Basic principle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |