silicates on:
[Wikipedia]
[Google]
[Amazon]
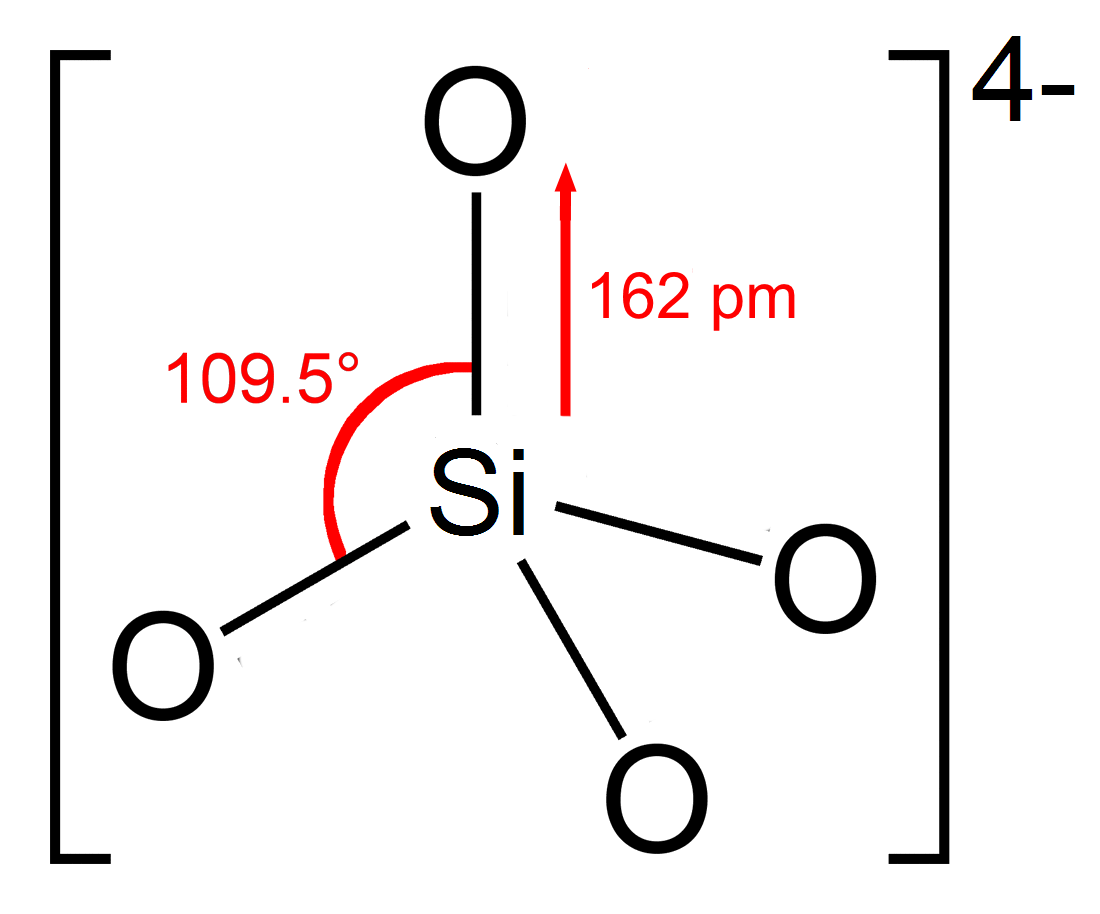 A silicate is any member of a family of polyatomic anions consisting of
A silicate is any member of a family of polyatomic anions consisting of

 With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate ring is a
With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate ring is a 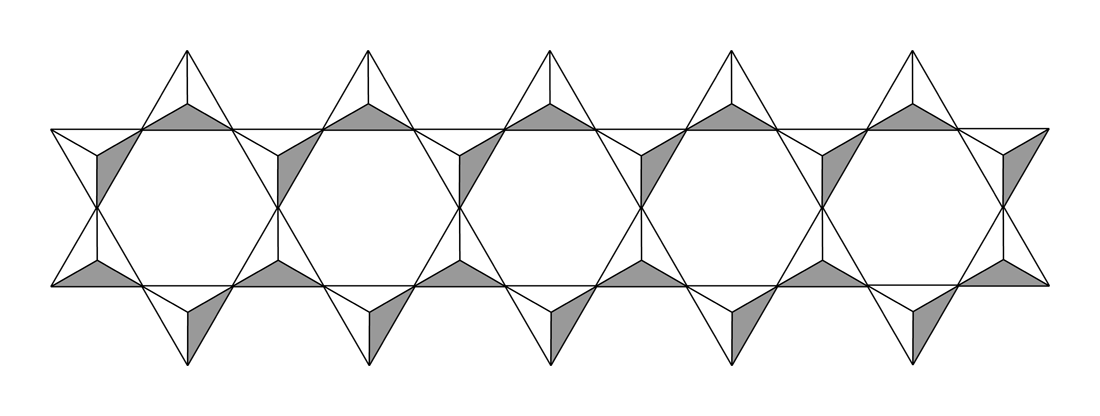 Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
 In this group, known as
In this group, known as
 A silicate is any member of a family of polyatomic anions consisting of
A silicate is any member of a family of polyatomic anions consisting of silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
, usually with the general formula , where . The family includes orthosilicate
In chemistry, orthosilicate is the anion , or any of its salts and esters. It is one of the silicate anions. It is occasionally called the silicon tetroxide anion or group.C. A. Kumins, and A. E. Gessler (1953), "Short-Cycle Syntheses of Ultr ...
(), metasilicate (), and pyrosilicate (, ). The name is also used for any salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
of such anions, such as sodium metasilicate
Sodium metasilicate is the chemical substance with formula , which is the main component of commercial sodium silicate solutions. It is an ionic compound consisting of sodium cations and the polymeric metasilicate anions ��–sub>''n''. It is ...
; or any ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate . Most commonly, silicates are encountered as silicate minerals
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, the crystalline forms of silica (silicon dio ...
.
For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite
Granite ( ) is a coarse-grained (phanerite, phaneritic) intrusive rock, intrusive igneous rock composed mostly of quartz, alkali feldspar, and plagioclase. It forms from magma with a high content of silica and alkali metal oxides that slowly coo ...
, gravel
Gravel () is a loose aggregation of rock fragments. Gravel occurs naturally on Earth as a result of sedimentation, sedimentary and erosion, erosive geological processes; it is also produced in large quantities commercially as crushed stone.
Gr ...
, and garnet
Garnets () are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
Garnet minerals, while sharing similar physical and crystallographic properties, exhibit a wide range of chemical compositions, de ...
) and artificial (such as Portland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in th ...
, ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcela ...
s, glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window pane ...
, and waterglass).
Structural principles
In most silicates, a silicon atom occupies the center of an idealizedtetrahedron
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
whose corners are four oxygen atoms, connected to it by single covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s according to the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
. The oxygen atoms, which bears some negative charge, link to other cations (Mn+). This Si-O-M-O-Si linkage is strong and rigid, which properties are manifested in the rock-like silicates. The silicates can be classified according to the length and crosslinking of the silicate anions.
Isolated silicates
Isolatedorthosilicate
In chemistry, orthosilicate is the anion , or any of its salts and esters. It is one of the silicate anions. It is occasionally called the silicon tetroxide anion or group.C. A. Kumins, and A. E. Gessler (1953), "Short-Cycle Syntheses of Ultr ...
anions have the formula . A common mineral in this group is olivine
The mineral olivine () is a magnesium iron Silicate minerals, silicate with the chemical formula . It is a type of Nesosilicates, nesosilicate or orthosilicate. The primary component of the Earth's upper mantle (Earth), upper mantle, it is a com ...
().
Two or more silicon atoms can share oxygen atoms in various ways, to form more complex anions, such as pyrosilicate .
Chains

 With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate ring is a
With two shared oxides bound to each silicon, cyclic or polymeric structures can result. The cyclic metasilicate ring is a hexamer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomer, monomers.Quote: ''Oligomer molecule: A molecule of intermediate ...
of SiO32-. Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
ic silicate anions of can exist also as long chains.
In single-chain silicates, which are a type of inosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, the crystalline forms of silica (silicon dio ...
, tetrahedra link to form a chain by sharing two oxygen atoms each. A common mineral in this group is pyroxene
The pyroxenes (commonly abbreviated Px) are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula , where X represents ions of calcium (Ca), sodium (Na), iron ( ...
.
 Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Double-chain silicates, the other category of inosilicates, occur when tetrahedra form a double chain (not always but mostly) by sharing two or three oxygen atoms each. Common minerals for this group are amphiboles.
Sheets
 In this group, known as
In this group, known as phyllosilicates
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, the crystalline forms of silica (silicon dio ...
, tetrahedra all share three oxygen atoms each and in turn link to form two-dimensional sheets. This structure does lead to minerals in this group having one strong cleavage plane. Micas
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into fragile elastic plates. This characteristic is described as ''perfect Cleavage (crystal), basal cle ...
fall into this group. Both muscovite
Muscovite (also known as common mica, isinglass, or potash mica) is a hydrated phyllosilicate mineral of aluminium and potassium with formula KAl2(Al Si3 O10)( F,O H)2, or ( KF)2( Al2O3)3( SiO2)6( H2O). It has a highly perfect basal cleavage y ...
and biotite
Biotite is a common group of phyllosilicate minerals within the mica group, with the approximate chemical formula . It is primarily a solid-solution series between the iron- endmember annite, and the magnesium-endmember phlogopite; more al ...
have very weak layers that can be peeled off in sheets.
Framework
In a framework silicate, known as atectosilicate
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust.
In mineralogy, the crystalline forms of silica (silicon dio ...
, each tetrahedron shares all 4 oxygen atoms with its neighbours, forming a 3D structure. Quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tet ...
and feldspar
Feldspar ( ; sometimes spelled felspar) is a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagiocl ...
s are in this group.
Silicates with non-tetrahedral silicon
Although the tetrahedron is a common coordination geometry for silicon(IV) compounds, silicon may also occur with higher coordination numbers. For example, in the anion hexafluorosilicate , the silicon atom is surrounded by sixfluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
atoms in an octahedral
In geometry, an octahedron (: octahedra or octahedrons) is any polyhedron with eight faces. One special case is the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at each vertex. Many types of i ...
arrangement. This structure is also seen in the hexahydroxysilicate anion that occurs in thaumasite, a mineral found rarely in nature but sometimes observed among other calcium silicate hydrate
Calcium silicate hydrates (CSH or C-S-H) are the main products of the hydration of Portland cement and are primarily responsible for the strength of cement-based materials. They are the main binding phase (the "glue") in most concrete. Only well de ...
s artificially formed in cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mi ...
and concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactur ...
structures submitted to a severe sulfate attack in argillaceous grounds containing oxidized
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
.
At very high pressure, such as exists in the majority of the Earth's rock, even SiO2 adopts the six-coordinated octahedral geometry in the mineral stishovite
Stishovite is an extremely hard, dense tetragonal form ( polymorph) of silicon dioxide. It is very rare on the Earth's surface; however, it may be a predominant form of silicon dioxide in the Earth, especially in the lower mantle.
Stishovite w ...
, a dense polymorph of silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
found in the lower mantle of the Earth and also formed by shock during meteorite
A meteorite is a rock (geology), rock that originated in outer space and has fallen to the surface of a planet or Natural satellite, moon. When the original object enters the atmosphere, various factors such as friction, pressure, and chemical ...
impacts.
Chemical properties
Silicates withalkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
cations and small or chain-like anions, such as sodium ortho- and metasilicate, are fairly soluble in water. They form several solid hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
s when crystallized from solution. Soluble sodium silicate
Sodium silicate is a generic name for chemical compounds with the formula or ·, such as sodium metasilicate (), sodium orthosilicate (), and sodium pyrosilicate (). The anions are often polymeric. These compounds are generally colorless tra ...
s and mixtures thereof, known as waterglass are important industrial and household chemicals. Silicates of non-alkali cations, or with sheet and tridimensional polymeric anions, generally have negligible solubility in water at normal conditions.
Reactions
Silicates are generally inert chemically. Hence they are common minerals. Their resiliency also recommends their use as building materials. When treated with calcium oxides and water, silicate minerals formPortland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in th ...
.
Equilibria involving hydrolysis of silicate minerals are difficult to study. The chief challenge is the very low solubility of SiO44- and its various protonated forms. Such equilibria are relevant to the processes occurring on geological time scales.G. B. Alexander (1953): "The Reaction of Low Molecular Weight Silicic Acids with Molybdic Acid". ''Journal of the American Chemical Society, volume 75, issue 22, pages 5655–5657. Some plants excrete ligands that dissolve silicates, a step in biomineralization
Biomineralization, also written biomineralisation, is the process by which living organisms produce minerals, often resulting in hardened or stiffened '' mineralized tissues''. It is an extremely widespread phenomenon: all six taxonomic kingd ...
.
Catechols can depolymerize SiO₂—a component of silicates with ionic structures like orthosilicate (SiO₄⁴⁻), metasilicate (SiO₂³⁻), and pyrosilicate (Si₂O₆⁷⁻)—by forming bis- and tris(catecholate)silicate dianions through coordination. This complexes can be further coated on various substrates for applications such as drug delivery systems, antibacterial and antifouling applications.
Detection
Silicate anions in solution react withmolybdate
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of +6: . Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, ...
anions yielding yellow silicomolybdate complexes. In a typical preparation, monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
ic orthosilicate was found to react completely in 75 seconds; dimeric pyrosilicate in 10 minutes; and higher oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relativ ...
s in considerably longer time. In particular, the reaction is not observed with suspensions of colloidal silica
Colloidal silicas are suspensions of fine amorphous, nonporous, and typically spherical silica particles in a liquid phase. It may be produced by Stöber process from Tetraethyl orthosilicate (TEOS).
Properties
Usually they are suspended in an ...
.
Zeolite formation and geopolymers polymerisation
The nature of soluble silicates is relevant to understandingbiomineralization
Biomineralization, also written biomineralisation, is the process by which living organisms produce minerals, often resulting in hardened or stiffened '' mineralized tissues''. It is an extremely widespread phenomenon: all six taxonomic kingd ...
and the synthesis of aluminosilicate
Aluminosilicate refers to materials containing anionic Si-O-Al linkages. Commonly, the associate cations are sodium (Na+), potassium (K+) and protons (H+). Such materials occur as minerals, coal combustion products and as synthetic materials, of ...
s, such as the industrially important catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s called zeolite
Zeolites are a group of several microporous, crystalline aluminosilicate minerals commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a meta ...
s. Along with aluminate
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminium atom.
Aluminate oxyanions
...
anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s, soluble silicate anions also play a major role in the polymerization mechanism of geopolymers. Geopolymers are amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
aluminosilicates whose production requires less energy than that of ordinary Portland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in th ...
. So, geopolymer cements could contribute to limiting the emissions in the Earth atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
and the global warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
caused by this greenhouse gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. T ...
.
See also
* * Alkali-silica reaction *Carbon cycle
The carbon cycle is a part of the biogeochemical cycle where carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of Earth. Other major biogeochemical cycles include the nitrogen cycle and the water cycl ...
* Carbonate-silicate cycle
* Ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
References
{{DEFAULTSORT:Silicon-oxygen tetrahedron Silicates Silicon oxyanions