|
HIV Vaccine
The HIV vaccine is a combination antiretroviral vaccine that protects people who are HIV-negative and at high risk of infection from HIV infection. According to the WHO, "every minute, one person in the world dies from AIDS-related causes." According to a UN report, there are about 39.9 million people living with HIV in the world, and 9.3 million (almost a quarter) are still not receiving life-saving treatment. The report states that if countries "take decisive action" to ensure funding and protect the rights of every person to protect themselves from HIV/AIDS, the number of people living with HIV and requiring lifelong treatment is projected to fall to 29 million by 2050. However, if no such action is taken, the number of people requiring lifelong treatment will increase to 46 million. Vaccine for HIV prevention In the United States, the Food and Drug Administration (FDA) has approved the first injectable HIV prevention drug for adults and adolescents, which only needs to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verified. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and recognize further and destroy any of the microorganisms associated with that agent that it may encounter in the future. Vaccines can be prophylaxis, prophylactic (to prevent or alleviate the effects of a future infection by a natural or "wild" pathogen), or therapeutic vaccines, therapeutic (to fight a disease that has already occurred, such as cancer vaccine, cancer). Some vaccines offer full sterilizing immunity, in which infection is prevented. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Health Organization
The World Health Organization (WHO) is a list of specialized agencies of the United Nations, specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Geneva, Switzerland, and has 6 regional offices and 150 field offices worldwide. Only sovereign states are eligible to join, and it is the largest intergovernmental health organization at the international level. The WHO's purpose is to achieve the highest possible level of health for all the world's people, defining health as "a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity." The main functions of the World Health Organization include promoting the control of epidemic and endemic diseases; providing and improving the teaching and training in public health, the medical treatment of disease, and related matters; and promoting the establishment of international standards for biologic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HIV Vaccine Development
An HIV vaccine is a potential vaccine that could be either a preventive vaccine or a therapeutic vaccine, which means it would either protect individuals from being infected with HIV or treat HIV-infected individuals. It is thought that an HIV vaccine could either induce an immune response against HIV (active vaccination approach) or consist of preformed antibodies against HIV (passive vaccination approach). Two active vaccine regimens, studied in the RV 144 and Imbokodo trials, showed they can prevent HIV in some individuals; however, the protection was in relatively few individuals, and was not long lasting. For these reasons, no HIV vaccines have been licensed for the market yet. Difficulties in development In 1984, after it was confirmed that HIV caused AIDS, the United States Health and Human Services Secretary Margaret Heckler declared that a vaccine would be available within two years. However, priming the adaptive immune system to recognize the viral envelope protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AIDS Vaccine
An HIV vaccine is a potential vaccine that could be either a preventive vaccine or a therapeutic vaccine, which means it would either protect individuals from being infected with HIV or treat HIV-infected individuals. It is thought that an HIV vaccine could either induce an immune response against HIV (active vaccination approach) or consist of preformed antibodies against HIV (passive vaccination approach). Two active vaccine regimens, studied in the RV 144 and Imbokodo trials, showed they can prevent HIV in some individuals; however, the protection was in relatively few individuals, and was not long lasting. For these reasons, no HIV vaccines have been licensed for the market yet. Difficulties in development In 1984, after it was confirmed that HIV caused AIDS, the United States Health and Human Services Secretary Margaret Heckler declared that a vaccine would be available within two years. However, priming the adaptive immune system to recognize the viral envelope proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AIDSVAX
AIDSVAX is an experimental HIV vaccine that was developed originally at Genentech in San Francisco, California, and later tested by the VaxGen company, a Genentech offshoot. The development and trials of the vaccine received significant coverage in the international media, but American trials proved inconclusive. The vaccine was then tested on a group of at-risk individuals in Thailand. In 1991, AIDSVAX originally consisted of the B envelope of recombinant gp120, a glycoprotein unique to HIV's surface, from a strain of the virus, MN, known at the time to infect people in the United States and Europe. The vaccine was designed to provoke the production of antibodies in subjects that would strip the gp120 protein off of the HIV viral particles, effectively disabling the virus so that it could not bind to or invade susceptible cells. Then, another group, infected with a second strain of HIV, A244, was discovered in 1995, and a revised, bivalent version of the vaccine was produced that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HIV Vaccine Trials Network
The HIV Vaccine Trials Network (HVTN) is a non-profit organization which connects physicians and scientists with activists and community educators for the purpose of conducting clinical trials seeking a safe and effective HIV vaccine. Collaboratively, researchers and layman, laypeople review potential vaccines for safety, immune response, and efficacy. The HVTN is a network for testing vaccines, and while its members may also work in vaccine development for other entities, the mission of the HVTN does not include vaccine design. The HVTN is the only HIV vaccine research network sponsored by the American government. It also manages the only large-scale HIV vaccine research trial network in Africa. The HVTN collaborates with the Division of Acquired Immunodeficiency Syndrome (DAIDS). Funding comes from the National Institute of Allergy and Infectious Diseases and National Institutes of Health, which oversee DAIDS. HVTN is headquartered at the Fred Hutchinson Cancer Research Center i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rilpivirine
Rilpivirine, sold under the brand name Edurant among others, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency (pharmacology), potency, longer biological half-life, half-life and reduced adverse drug reaction, side-effect profile compared with older NNRTIs such as efavirenz. Medical uses In the US, rilpivirine is approved for treatment-naive patients with a viral load of 100,000 copies/mL or less at therapy initiation. It has to be combined with other drugs against HIV. In the European Union, rilpivirine is approved in combination with cabotegravir for maintenance treatment of adults who have undetectable HIV levels in the blood (viral load less than 50 copies/ml) with their current antiretroviral treatment, and when the virus has not developed resistance to certain class of anti-HIV medicines called non-nucleoside reverse transcriptase inhibitors (NNRTI ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cabenuva
Cabotegravir/rilpivirine, sold under the brand name Cabenuva, is a co-packaged antiretroviral medication for the treatment of HIV/AIDS. It contains cabotegravir and rilpivirine in a package with two separate injection vials. The most common adverse reactions include injection site reactions, fever or feeling hot (pyrexia), fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness and rash. The co-packaged medication was approved for medical use in the United States in January 2021. It is the first FDA-approved injectable, complete regimen for HIV-infected adults that is administered once a month. It is also approved for use in Canada. In the European Union, the two medications are approved separately and have different brand names: Vocabria (for cabotegravir) and Rekambys (for rilpivirine). Medical uses Cabotegravir/rilpivirine is indicated as a complete regimen for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults to repl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ViiV Healthcare
ViiV Healthcare ( ) is a British multinational pharmaceutical company specializing in the research and development of medicines to treat and prevent HIV/AIDS. Its global headquarters is located in London. The company was created as a joint venture by GSK and Pfizer in November 2009, with both companies transferring their HIV assets to the new company.Jacks, Andre"GSK and Pfizer to Merge HIV PortfoliosFinancial Times. 16 April 2010 In 2012, Shionogi joined the company. As of December 2023, 76.5% of the company is owned by GSK, 13.5% by Pfizer and 10% by Shionogi. According to The Financial Times, the company's co-ownership structure may change depending on the achievement of certain milestones. ViiV Healthcare's products have a market share of approximately 32% of the global HIV therapy market, making it the second-largest healthcare company in the sector, after Gilead Sciences. ViiV Healthcare's global headquarters are in London in the United Kingdom, and the company h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
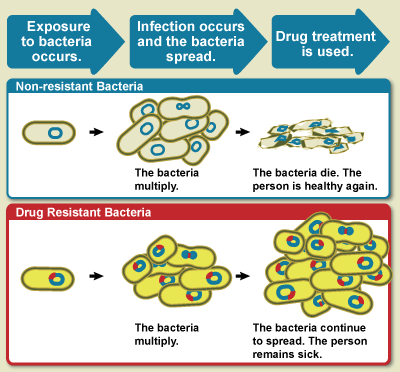
Drug Resistance
Drug resistance is the reduction in effectiveness of a medication such as an antimicrobial or an antineoplastic in treating a disease or condition. The term is used in the context of resistance that pathogens or cancers have "acquired", that is, resistance has evolved. Antimicrobial resistance and antineoplastic resistance challenge clinical care and drive research. When an organism is resistant to more than one drug, it is said to be multidrug-resistant. The development of antibiotic resistance in particular stems from the drugs targeting only specific bacterial molecules (almost always proteins). Because the drug is ''so'' specific, any mutation in these molecules will interfere with or negate its destructive effect, resulting in antibiotic resistance. Furthermore, there is mounting concern over the abuse of antibiotics in the farming of livestock, which in the European Union alone accounts for three times the volume dispensed to humans – leading to development of super-re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pre-exposure Prophylaxis For HIV Prevention
Pre-exposure prophylaxis for HIV prevention, commonly known as PrEP, is the use of antiviral drugs as a strategy for the prevention of HIV/AIDS by people that do not have HIV/AIDS. PrEP is one of a number of HIV prevention strategies for people who are HIV-negative but who have a higher risk of acquiring HIV, including sexually-active adults who are at increased risk of contracting HIV, people who engage in intravenous drug use (see drug injection), and serodiscordant sexually-active couples. The first form of PrEP for HIV prevention—Emtricitabine/tenofovir, emtricitabine and tenofovir disoproxil (FTC/TDF; ''Truvada'')—was approved in 2012. In October 2019, the U.S. Food and Drug Administration (FDA) approved the combination of emtricitabine and tenofovir alafenamide (FTC/TAF; ''Descovy'') to be used as PrEP in addition to Truvada, which provides similar levels of protection. ''Descovy'', however, is currently approved only for cisgender males and transgender women as the ef ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiretroviral
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same-sex and opposite-sex partners so long as the HIV-positive partner maintains an undetectable viral load. Treatment has been so successful that in many parts of the world, HIV has become a chronic condition in which progression to AIDS is increasingly rare. Anthony Fauci, former head of the United States National Institute of Allergy and Infectious Diseases, has written, "With collective and resolute action ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |