|
HCCCN
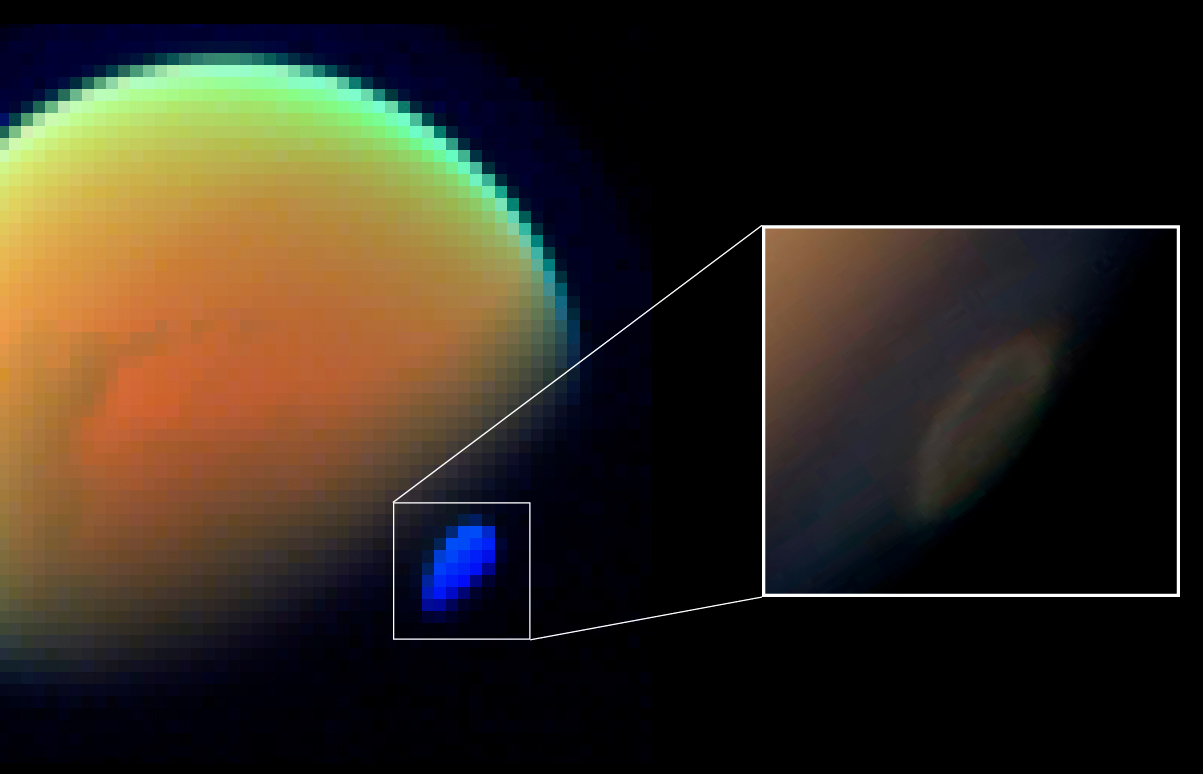
Cyanoacetylene is an organic compound with the formula or . It is the simplest cyanopolyyne. Cyanoacetylene has been detected by spectroscopic methods in interstellar clouds, in the coma of comet Hale–Bopp and in the atmosphere of Saturn's moon Titan, where it sometimes forms expansive fog-like clouds. Cyanoacetylene is one of the molecules that was produced in the Miller–Urey experiment. : Nickel carbonyl catalyzes cyanoacetylene carboalkoxylation to cyanoacrylate esters. See also *Dicyanoacetylene, N≡C−C≡C−C≡N *Diacetylene, H−C≡C−C≡C−H *Cyanogen, N≡C−C≡N *Hydrocyanic acid, H−C≡N *Polyyne A polyyne is any organic compound with alternating Single bond, single and triple bonds; that is, a series of consecutive alkynes, with ''n'' greater than 1. These compounds are also called polyacetylenes, especially in the natural products and ..., R−(C≡C)''n''−R References {{Authority control Alkyne derivatives Conjugated nitriles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Carbonyl
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a Organonickel chemistry, nickel(0) organometallic compound with the chemical formula, formula Ni(CO)4. This colorless liquid is the principal metal carbonyl, carbonyl of nickel. It is an Reaction intermediate, intermediate in the Mond process for producing very high-purity nickel and a reagent in organometallic chemistry, although the Mond Process has fallen out of common usage due to the health hazards in working with the compound. Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption. Structure and bonding In nickel tetracarbonyl, the oxidation state for nickel is assigned as zero, because the Ni−C bonding electrons come from the C atom and are still assigned to C in the hypothetical ionic bond which determines the oxidation states. The formula conforms to the 18-electron rule. The molecule is tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyyne
A polyyne is any organic compound with alternating Single bond, single and triple bonds; that is, a series of consecutive alkynes, with ''n'' greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds with ''n'' greater than 1. They are also sometimes referred to as oligoynes, or carbinoids after "linear acetylenic carbon, carbyne" , the hypothetical allotrope of carbon that would be the ultimate member of the series. In ''Avancés récentes en chimie des acétylènes – Recent advances in acetylene chemistry'' The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today. The simplest polyyne is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocyanic Acid
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the formula HCN and structural formula . It is a highly toxic and flammable liquid that boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. A solution of hydrogen cyanide in water, represented as HCN( aq), is called ''hydrocyanic acid''. The salts of the cyanide anion are known as cyanides. Whether hydrogen cyanide is an organic compound or not is a topic of debate among chemists, and opinions vary from author to author. Traditionally, it is considered inorganic by a significant number of authors. Contrary to this view, it is considered organic by other authors, because ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen
Cyanogen is the chemical compound with the chemical formula, formula . Its structure is . The simplest stable carbon nitride, it is a Transparency and translucency, colorless and highly toxic gas with a pungency, pungent odor. The molecule is a pseudohalogen. Cyanogen molecules are linear molecular geometry, linear, and consist of two CN groups ‒ analogous to diatomic halogen molecules, such as chlorine, Cl, but far less oxidizing. The two cyanide, cyano groups are bonded together at their carbon atoms, though other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide () (but see also ''Cyano radical''). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene). Cyanogen is the anhy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diacetylene
Diacetylene (also known as butadiyne) is the organic compound with the formula or . It is the simplest compound containing two triple bonds. It is first in the series of polyynes, which are of theoretical but not of practical interest. Occurrence Diacetylene has been identified in the atmosphere of Titan and in the protoplanetary nebula CRL 618 by its characteristic vibrational spectrum. It is proposed to arise by a reaction between acetylene and the ethynyl radical (), which is produced when acetylene undergoes photolysis. This radical can in turn attack the triple bond in acetylene and react efficiently even at low temperatures. Diacetylene has also been detected on the Moon. Preparation This compound may be made by the dehydrohalogenation of 1,4-dichloro-2-butyne by potassium hydroxide (in alcoholic medium) at ~70°C: : The bis(trimethylsilyl)-protected derivative may be prepared by the Hay coupling of (trimethylsilyl)acetylene: : See also * Acetylene * Diiodobutadi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicyanoacetylene
Dicyanoacetylene, also called carbon subnitride or but-2-ynedinitrile (IUPAC), is a compound of carbon and nitrogen with chemical formula . At room temperature, dicyanoacetylene is a colorless volatile liquid. It has a linear molecular structure, (often abbreviated as ), with alternating triple and single covalent bonds. It can be viewed as acetylene with the two hydrogen atoms replaced by cyanide groups. Because of its high endothermic heat of formation, dicyanoacetylene can explode to carbon powder and nitrogen gas, : and it burns in oxygen with a bright blue-white flame at a temperature of , the hottest flame in oxygen; burned in ozone at high pressure the flame temperature exceeds . Dicyanoacetylene polymerizes at room temperature into a dark solid. Synthesis Dicyanoacetylene can be prepared by passing nitrogen gas over a sample of graphite heated to temperatures between . It may also be synthesized via a reaction between a dihaloacetylene and a cyanide salt: : As a r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanoacrylate
Cyanoacrylates are a family of strong fast-acting adhesives with industrial, medical, and household uses. They are derived from ethyl cyanoacrylate and related esters. The cyanoacrylate group in the monomer rapidly polymerizes in the presence of water to form long, strong chains. Specific cyanoacrylates include methyl cyanoacrylate, methyl 2-cyanoacrylate (MCA), ethyl cyanoacrylate, ethyl 2-cyanoacrylate (ECA, commonly sold under trade names such as "Super Glue" and "Krazy Glue"), butyl cyanoacrylate, ''n''-butyl cyanoacrylate (n-BCA), octyl cyanoacrylate, and 2-octyl cyanoacrylate (used in medical, veterinary and first aid applications). Cyanoacrylate adhesives are sometimes known generically as instant glue, power glue, or super glue. The abbreviation "CA" is commonly used for industrial grade cyanoacrylate. Development The original patent for cyanoacrylate was filed in 1947 by the B.F. Goodrich Company as an outgrowth of a search for materials suitable for clear plastic Si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboalkoxylation
In industrial chemistry, carboalkoxylation is a process for converting alkenes to esters. This reaction is a form of carbonylation. A closely related reaction is hydrocarboxylation, which employs water in place of alcohols. A commercial application is the carbomethoxylation of ethylene to give methyl propionate: : The process is catalyzed by . Under similar conditions, other Pd-diphosphines catalyze formation of polyethyleneketone. Methyl propionate ester is a precursor to methyl methacrylate, which is used in plastics and adhesives. Carboalkoxylation has been incorporated into various telomerization schemes. For example carboalkoxylation has been coupled with the dimerization of 1,3-butadiene. This step produces a doubly unsaturated C9-ester: : Hydroesterification Related to carboalkoxylation is hydroesterification, the insertion of alkenes and alkynes into the H-O bond of carboxylic acids. Vinyl acetate is produced industrially by the addition of acetic acid to acetylene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Miller–Urey Experiment
The Miller–Urey experiment, or Miller experiment, was an experiment in chemical synthesis carried out in 1952 that simulated the conditions thought at the time to be present in the Prebiotic atmosphere, atmosphere of the early, prebiotic Earth. It is seen as one of the first successful experiments demonstrating the synthesis of organic compounds from inorganic compound, inorganic constituents in an Abiogenesis, origin of life scenario. The experiment used methane (CH4), ammonia (NH3), hydrogen (H2), in ratio 2:1:2, and water (H2O). Applying an electric arc (simulating lightning) resulted in the production of amino acids. It is regarded as a groundbreaking experiment, and the classic experiment investigating the origin of life (abiogenesis). It was performed in 1952 by Stanley Miller, supervised by Nobel laureate Harold Urey at the University of Chicago, and published the following year. At the time, it supported Alexander Oparin's and J. B. S. Haldane's hypothesis that the cond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanopolyyne
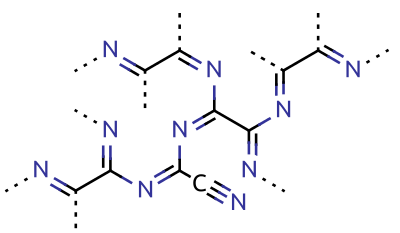
In organic chemistry, cyanopolyynes are a family of organic compounds with the chemical formula (''n'' = 3,5,7,…) and the structural formula (''n'' = 1,2,3,…). Structurally, they are polyynes with a cyano group () covalently bonded to one of the terminal acetylene units (). A rarely seen group of molecules both due to the difficulty in production and the unstable nature of the paired groups, the cyanopolyynes have been observed as a major organic component in interstellar clouds. This is believed to be due to the hydrogen scarcity of some of these clouds. Interference with hydrogen is one of the reason for the molecule's instability due to the energetically favorable dissociation back into hydrogen cyanide and acetylene. Cyanopolyynes were first discovered in interstellar molecular clouds in 1971 using millimeter wave and microwave telescopes. Since then many higher weight cyanopolyynes such as and have been discovered, although some of these i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features Peer review, peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2022 ''Journal Citation Reports'' (with an ascribed impact factor of 50.5), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in the autumn of 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander MacMillan (publisher), Alexander MacMillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |