|
Glycoprotein Metabolism Disorders
Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-GlcNAc
''O''-GlcNAc (short for ''O''-linked GlcNAc or ''O''-linked β-''N''-acetylglucosamine) is a reversible Enzyme, enzymatic post-translational modification that is found on serine and threonine residues of Cell nucleus, nucleoCytoplasm, cytoplasmic proteins. The modification is characterized by a Glycosidic bond, β-glycosidic bond between the Hydroxy group, hydroxyl group of serine or threonine side chains and N-Acetylglucosamine, ''N''-acetylglucosamine (GlcNAc). ''O''-GlcNAc differs from other forms of protein glycosylation: (i) ''O''-GlcNAc is not elongated or modified to form more complex glycan structures, (ii) ''O''-GlcNAc is almost exclusively found on nuclear and cytoplasmic proteins rather than membrane proteins and secretory proteins, and (iii) ''O''-GlcNAc is a highly dynamic modification that turns over more rapidly than the proteins which it modifies. ''O''-GlcNAc is conserved across Animal, metazoans. Due to the dynamic nature of ''O''-GlcNAc and its presence on seri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoserine
Phosphoserine (abbreviated as SEP or J) is an ester of serine and phosphoric acid. Phosphoserine is a component of many proteins as the result of posttranslational modifications. The phosphorylation of the alcohol functional group in serine to produce phosphoserine is catalyzed by various types of kinases. Through the use of technologies that utilize an expanded genetic code, phosphoserine can also be incorporated into proteins during translation. It is a normal metabolite In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ... found in human biofluids. Phosphoserine has three potential coordination sites (carboxyl, amine and phosphate group) Determination of the mode of coordination between phosphorylated ligands and metal ions occurring in an organism is a first step to explain the fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorous Acid
Phosphorous acid (or phosphonic acid) is the Compound (chemistry), compound described by the chemical formula, formula . It is diprotic (readily ionizes two protons), not triprotic as might be suggested by its formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula , are called phosphonic acids. Nomenclature and tautomerism Solid has tetrahedral geometry about the central phosphorus atom, with a bond of 132 picometer, pm, one double bond of 148 pm and two longer single bonds of 154 pm. In common with other phosphorus oxides with bonds (e.g.hypophosphorous acid and dialkyl phosphites), it exists in equilibrium with an extremely minor tautomer . (In contrast, arsenous acid's major tautomer is the trihydroxy form.) IUPAC recommends that the trihydroxy form be called phosphorous acid, and the dihydroxy form phosphonic acid.. Only the reduced phosphorus c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
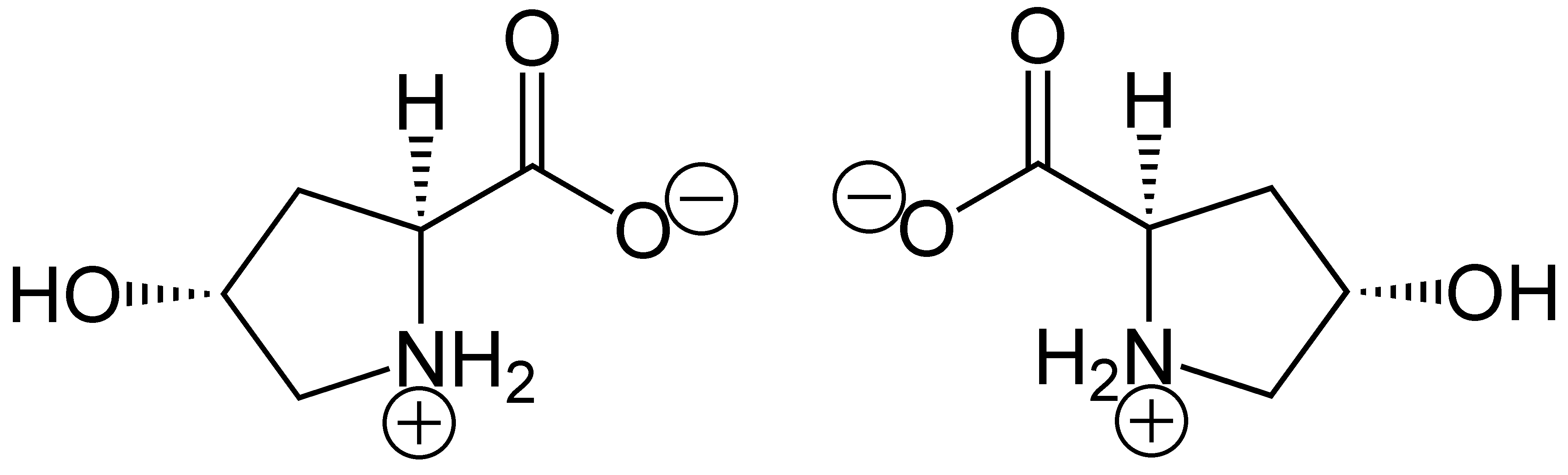
Hydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank. Structure and discovery In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelatin. In 1905, Hermann Leuchs synthesized a racemic mixture of 4-hydroxyproline. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom. Production and function Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl 4-hydroxylase following protein synthesis (as a post-translational modification). The enzyme-catalyzed reaction takes place in the lumen of the endoplasmic reticulum. Although it is not directly incorporated into proteins, hydroxyproline comprises roughly 4% of all amino acids found in animal tissue, an amount greater than seven other amino acids that are translationally incorporated. Animals Collagen Hydroxyproline is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylysine
Hydroxylysine (Hyl) is an amino acid with the molecular formula C6H14N2O3. It was first discovered in 1921 by Donald Van Slyke as the 5-hydroxylysine form. It arises from a post-translational hydroxy modification of lysine. It is most widely known as a component of collagen Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a trip .... It is biosynthesized from lysine via oxidation by lysyl hydroxylase enzymes. The most common form is the (5''R'') stereoisomer found in collagen. However, the enzyme JMJD6 has recently been shown to be a lysyl hydroxylase which modifies an RNA splicing factor producing the (5''S'') stereoisomer. Additionally, in ''E. coli'', there has been at least one lysine ''N''-hydroxylase enzyme identified, named IucD. References External links * {{MeshName, Hydrox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. The one-letter symbol Y was assigned to tyrosine for being alphabetically nearest of those letters available. Note that T was assigned to the structurally simpler threonine, U was avoided for its similarity with V for valine, W was assigned to tryptophan, while X was reserved for undetermined or atypical amino acids. The mnemonic t''Y''rosine was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− form when dissolved in water), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the proteinogenic amino acids. Only the L- stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, '' sericum''. Serine's structure was established in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-linked Glycosylation
''O''-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. ''O''-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |