|
Glowing With You
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emitti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive Decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opalescence
Opalescence or play of color is an optical phenomenon associated with the mineraloid gemstone opal,opalescent. 2019. In Noah Webster's 1828 American Dictionary of the English Language. Retrieved January 7, 2019, from https://1828.mshaffer.com/d/word/opalescent a hydrated silicon dioxide. This effect appears as a milky, translucent glow that changes with the angle of light, often creating a soft, pearly sheen that can display various colors or hues. Opalescence can be seen in materials like certain minerals, glass, and even fluids. Definition Each of the three notable types of opalprecious, common, and fireDouma, M., curator. 2008. Opal. In Cause of Color. Retrieved January 8, 2019, from https://webexhibits.org/causesofcolor/15F.htmldisplay different optical effects; therefore, the intended meaning varies depending on context. * The general definition of ''opalescence'' is a milky iridescence displayed by an opal, which describes the visual effect of precious opal very well ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
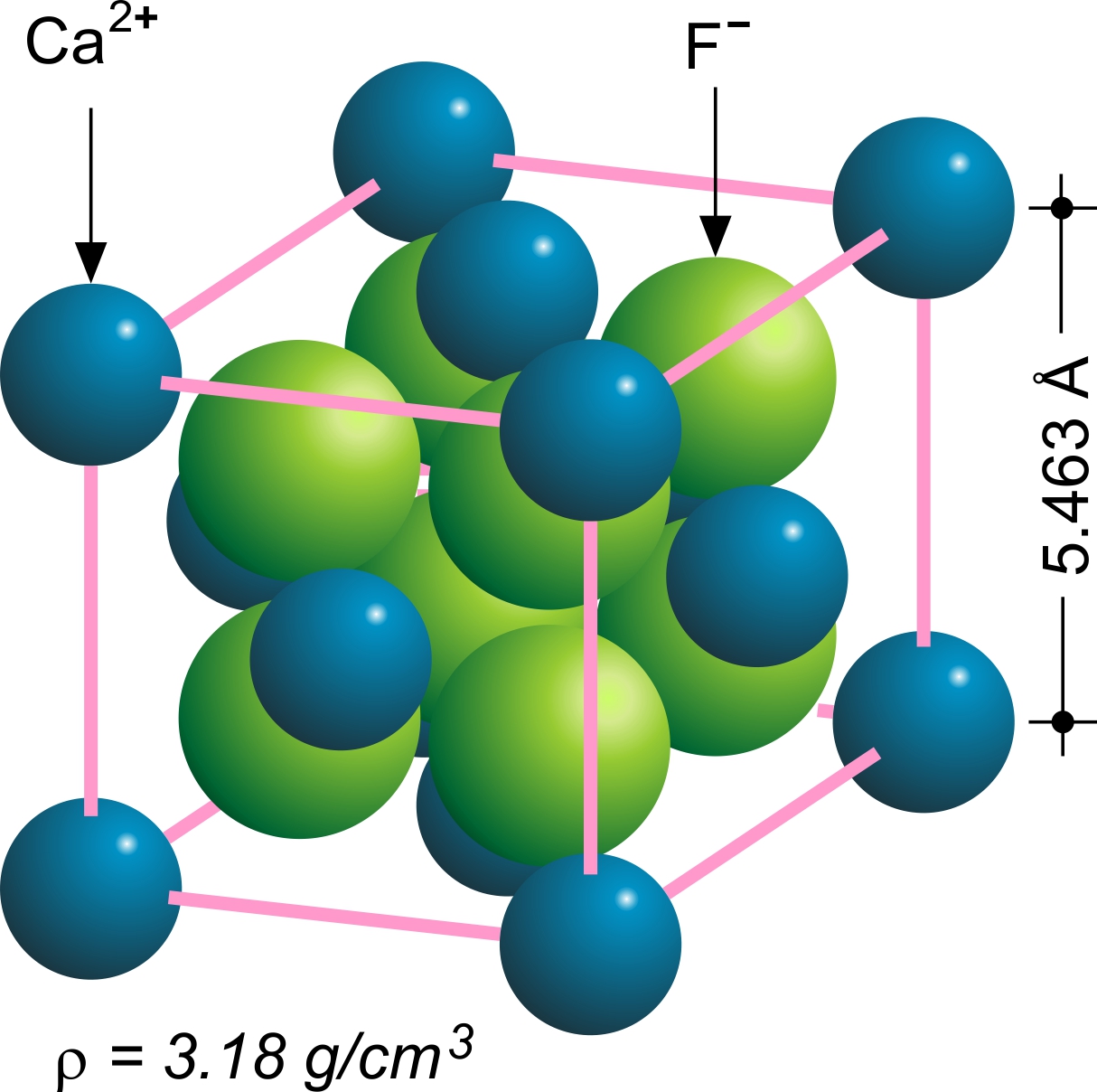
Fluorspar
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
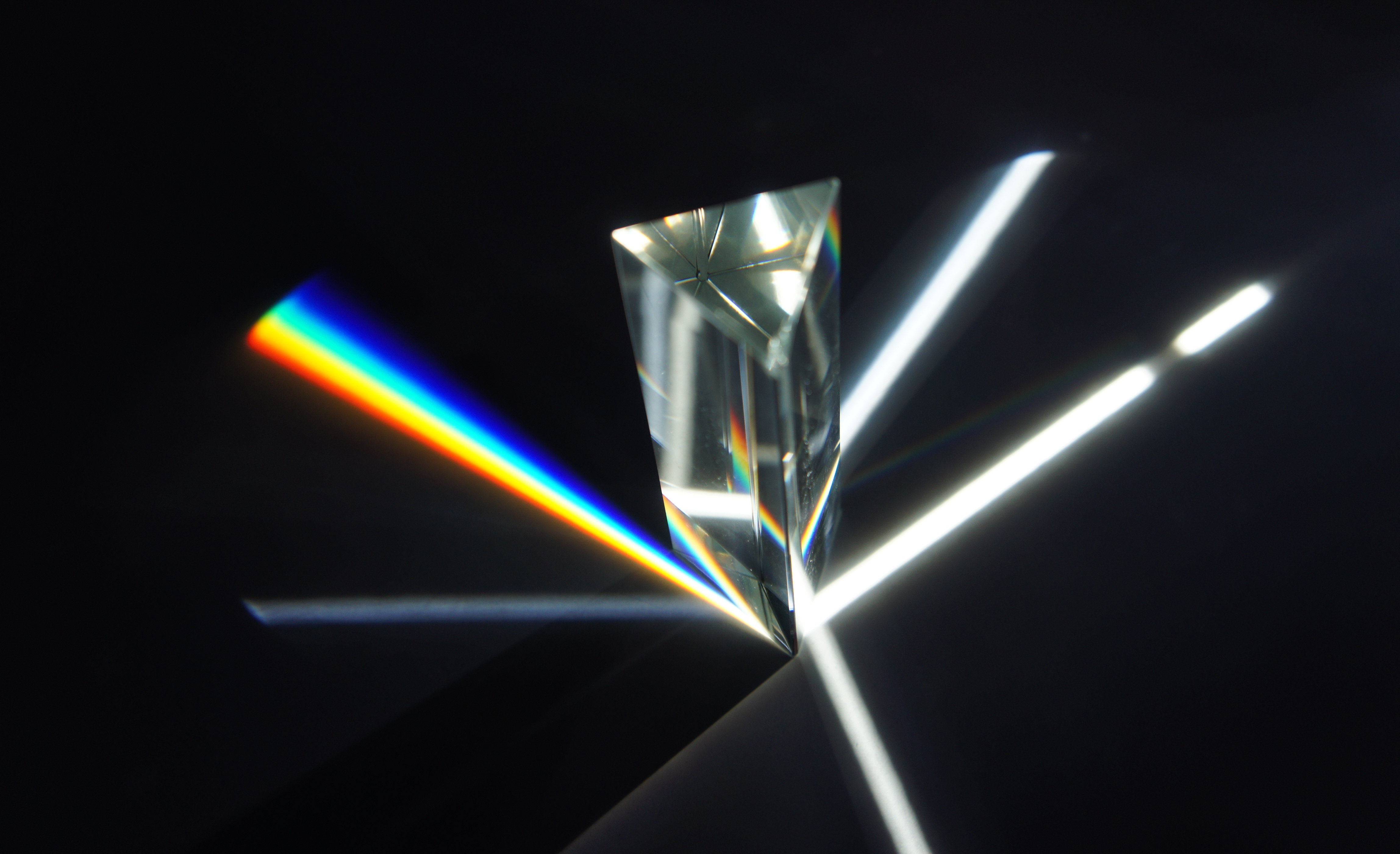
Prism (optics)
An optical prism is a transparent optical element with flat, polished surfaces that are designed to refract light. At least one surface must be angled—elements with two parallel surfaces are ''not'' prisms. The most familiar type of optical prism is the triangular prism, which has a triangular base and rectangular sides. Not all optical prisms are geometric prisms, and not all geometric prisms would count as an optical prism. Prisms can be made from any material that is transparent to the wavelengths for which they are designed. Typical materials include glass, acrylic and fluorite. A dispersive prism can be used to break white light up into its constituent spectral colors (the colors of the rainbow) to form a spectrum as described in the following section. Other types of prisms noted below can be used to reflect light, or to split light into components with different polarizations. Types Dispersive ''Dispersive prisms'' are used to break up light into its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinine Sulfate
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to ''Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg cramps, quinine is not recommended for this purpose due to the risk of serious side effects. It can be taken by mouth or intravenously. Malaria resistance to quinine occurs in certain areas of the world. Quinine is also used as an ingredient in tonic water and other beverages to impart a bitter taste. Common side effects include headache, ringing in the ears, vision issues, and sweating. More severe side effects include deafness, low blood platelets, and an irregular heartbeat. Use can make one more prone to sunburn. While it is unclear if use during pregnancy carries potential for fetal harm, treating malaria during pregnancy with quinine when appropriate is still recommended. Quinine is an alkaloid, a naturally occurring chemical c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sir George Stokes, 1st Baronet
Sir George Gabriel Stokes, 1st Baronet, (; 13 August 1819 – 1 February 1903) was an Irish mathematician and physicist. Born in County Sligo, Ireland, Stokes spent his entire career at the University of Cambridge, where he served as the Lucasian Professor of Mathematics for 54 years, from 1849 until his death in 1903, the longest tenure held by the Lucasian Professor. As a physicist, Stokes made seminal contributions to fluid mechanics, including the Navier–Stokes equations; and to physical optics, with notable works on polarisation and fluorescence. As a mathematician, he popularised "Stokes' theorem" in vector calculus and contributed to the theory of asymptotic expansions. Stokes, along with Felix Hoppe-Seyler, first demonstrated the oxygen transport function of haemoglobin, and showed colour changes produced by the aeration of haemoglobin solutions. Stokes was made a baronet by the British monarch in 1889. In 1893 he received the Royal Society's Copley Medal, then the mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic compound, organic and inorganic compound, inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the Phosphorus (morning star), god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hennig Brand
Hennig Brand (; or ) was a German alchemist who lived and worked in Hamburg. In 1669, Brand accidentally discovered the chemical element phosphorus while searching for the "philosopher's stone", a substance which was believed to transmute base metals into gold. Biography The circumstances of Brand's birth are unknown but he was born in 1630 and died or 1710. Some sources describe his origins as humble and indicate that he had been an apprentice glassmaker as a young man. However, correspondence by his second wife Margaretha states that he was of high social standing. In any case he held a post as a junior army officer during the Thirty Years' War and his first wife's dowry was substantial, allowing him to pursue alchemy on leaving the army. He was one of the many searchers for the philosopher's stone. In the process, he accidentally discovered phosphorus. Discovery of phosphorus Like other alchemists of the time, Brand searched for the "philosopher's stone", a substance whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcination
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition. The root of the word calcination refers to its most prominent use, which is to remove carbon from limestone (calcium carbonate) through combustion to yield calcium oxide (quicklime). This calcination reaction is CaCO3(s) → CaO(s) + CO2(g). Calcium oxide is a crucial ingredient in modern cement, and is also used as a chemical flux in smelting. Industrial calcination generally emits carbon dioxide (). A calciner is a steel cylinder that rotates inside a heated furnace and performs indirect high-temperature processing (550–1150 °C, or 1000–2100 °F) within a controlled atmosphere. Etymology The process of calcination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
De Phenomenis In Orbe Lunae
''De Phenomenis in Orbe Lunae'' is a 1612 book by Collegio Romano philosophy professor Giulio Cesare la Galla that describes emission of light by a stone. La Galla's inspiration came from Galileo's debate with Vincenzo Casciarolo regarding a " lapis solaris," a stone that emitted light seemingly on its own. In ''De Phenomenis'', de Galla asserts that the stone was only able to emit light after the stone itself had calcified. It released "a certain quantity of fire and light" that it had absorbed, just as water would be absorbed by a sponge. Robert Burton discusses ''De Phenomenis'' in ''The Anatomy of Melancholy ''The Anatomy of Melancholy'' (full title: ''The Anatomy of Melancholy, What it is: With all the Kinds, Causes, Symptomes, Prognostickes, and Several Cures of it. In Three Maine Partitions with their several Sections, Members, and Subsections. Ph ....'' References 1612 books Astronomy books {{astronomy-book-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |