|
Generic Pharmaceutical Association
The Association for Accessible Medicines (AAM), Washington, D.C., is a trade association representing the manufacturers and distributors of generic prescription drugs, manufacturers and distributors of bulk pharmaceutical chemicals, and suppliers of other goods and services to the U.S. generic drug industry. As the primary lobby for makers of generic drugs, AAM's stated mission is to advocate for public policies that facilitate timely access to lower-cost, FDA-approved generic and biosimilar medicines by consumers and patients. Over the 10-year period 2008 through 2018, the use of generic drugs generated $2 trillion in U.S. healthcare savings. Prior to February 2017, AAM was the Generic Pharmaceutical Association (GPhA). History GPhA was formed in May 2000 by the merger of the Generic Pharmaceutical Industry Association (GPIA) and the National Pharmaceutical Alliance (NPA). In January 2001, the other generic industry trade association in operation at the time, the National Associa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trade Association
A trade association, also known as an industry trade group, business association, sector association or industry body, is an organization founded and funded by businesses that operate in a specific Industry (economics), industry. An industry trade association participates in public relations activities such as advertising, education, publishing, lobbying, and political donations, but its focus is collaboration between companies. Associations may offer other services, such as producing conferences, holding networking or charitable events, or offering classes or educational materials. Many associations are non-profit organizations governed by bylaws and directed by officers who are also members. In countries with a social market economy, the role of trade associations is often taken by employers' organizations, which also take a role in social dialogue. Political influence One of the primary purposes of trade groups, particularly in the United States, is to attempt to influence p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
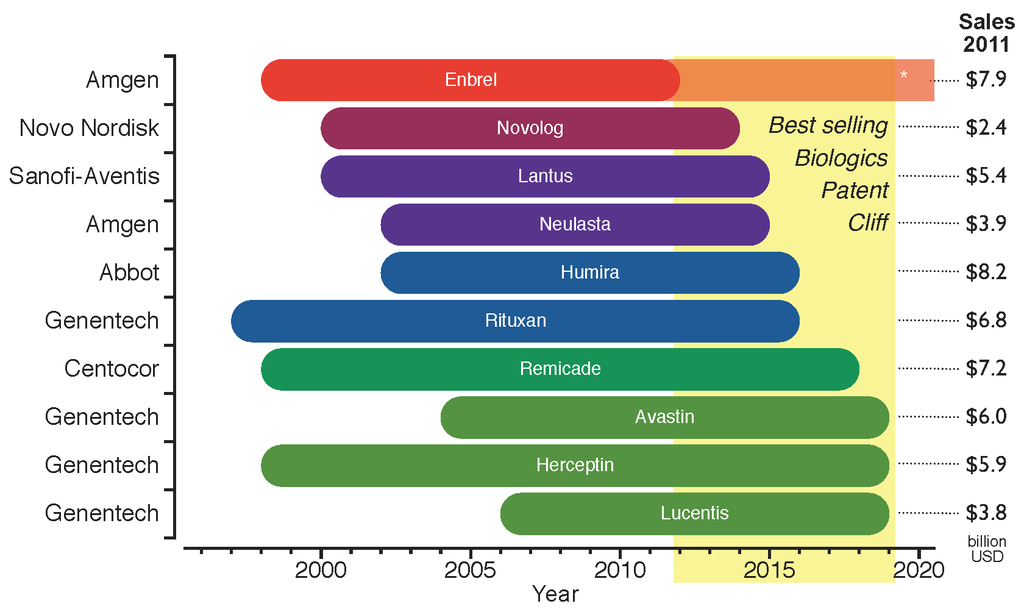
Biologics Price Competition And Innovation Act
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They (or their precursors or components) are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine. Terminology surrounding biopharmaceuticals varies between groups and entities, with different terms referring to different subsets of therapeutics within the general biopharmaceutical category. Some regulatory agencies use the terms ''biologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lobbying Organizations In The United States
In politics, lobbying, persuasion or interest representation is the act of lawfully attempting to influence the actions, policies, or decisions of government officials, most often legislators or members of regulatory agencies. Lobbying, which usually involves direct, face-to-face contact, is done by many types of people, associations and organized groups, including individuals in the private sector, corporations, fellow legislators or government officials, or advocacy groups (interest groups). Lobbyists may be among a legislator's constituencies, meaning a voter or bloc of voters within their electoral district; they may engage in lobbying as a business. Professional lobbyists are people whose business is trying to influence legislation, regulation, or other government decisions, actions, or policies on behalf of a group or individual who hires them. Individuals and nonprofit organizations can also lobby as an act of volunteering or as a small part of their normal job. Governm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contract Research Organization
In the life sciences, a contract research organization (CRO) is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. A CRO may provide such services as biopharmaceutical development, biological assay development, commercialization, clinical development, clinical trials management, pharmacovigilance, outcomes research, and Real world evidence. CROs are designed to reduce costs for companies developing new medicines and drugs in niche markets. They aim to simplify entry into drug markets, and simplify development, as the need for large pharmaceutical companies to do everything ‘in house’ is now redundant. CROs also support foundations, research institutions, and universities, in addition to governmental organizations (such as the NIH, EMA, etc.). Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. However, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicines For Europe
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generic Drug User Fee Act
Generic or generics may refer to: In business * Generic term, a common name used for a range or class of similar things not protected by trademark * Generic brand, a brand for a product that does not have an associated brand or trademark, other than the trading name of the business providing the product * Generic trademark, a trademark that sometimes or usually replaces a common term in colloquial usage * Generic drug, a drug identified by its chemical name rather than its brand name In computer programming * Generic function, a computer programming entity made up of all methods having the same name * Generic programming, a computer programming paradigm based on method/functions or classes defined irrespective of the concrete data types used upon instantiation ** Generics in Java In linguistics *A pronoun or other word used with a less specific meaning, such as: ** generic ''you'' ** generic ''he'' or generic ''she'' ** generic ''they'' * Generic mood, a grammatical mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patient Protection And Affordable Care Act
The Affordable Care Act (ACA), formally known as the Patient Protection and Affordable Care Act and colloquially known as Obamacare, is a landmark U.S. federal statute enacted by the 111th United States Congress and signed into law by President Barack Obama on March 23, 2010. Together with the Health Care and Education Reconciliation Act of 2010 amendment, it represents the U.S. healthcare system's most significant regulatory overhaul and expansion of coverage since the enactment of Medicare and Medicaid in 1965. The ACA's major provisions came into force in 2014. By 2016, the uninsured share of the population had roughly halved, with estimates ranging from 20 to 24 million additional people covered. The law also enacted a host of delivery system reforms intended to constrain healthcare costs and improve quality. After it went into effect, increases in overall healthcare spending slowed, including premiums for employer-based insurance plans. The increased coverage was d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bruce Downey
Bruce Downey is the former chairman and CEO of Barr Pharmaceuticals (bought by Teva Pharmaceuticals) and in 2009 became a partner at New Spring Capital, a venture capital firm. Career Downey began his career in the Honors Program of the U.S. Department of Justice. He later worked as a special litigation counsel at the U.S. Department of Energy and then became a partner at the law firm of Winston & Strawn. In January 1993, Downey was appointed president and chief operating officer of Barr Pharmaceuticals. He became chairman and CEO in 1994 when Barr's founder Edwin A. Cohen stepped down. During Downey's tenure at Barr, the company grew revenue from $60 million to more than $2.5 billion and became the fourth largest global generic drug company. In 2008, Barr Pharmaceuticals was acquired by Teva Pharmaceutical. Downey was named Ernst & Young Entrepreneur of the Year in 2009. Downey is a member of the board of directors for Cardinal Health and Momenta Pharmaceuticals. He also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generic Drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging. Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries in which they are dispensed. They are labeled with the name of the manufacturer and a generic non-proprietary name such as the United States Adopted Name (USAN) or International Nonproprietary Name (INN) of the drug. A generic drug must contain the same active ingredients as the original brand- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosimilar
A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company. Biosimilars are officially approved versions of original "innovator" products and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval. Unlike with generic drugs of the more common small-molecule type, biologics generally exhibit high molecular complexity and may be quite sensitive to changes in manufacturing processes. Despite that heterogeneity, all biopharmaceuticals, including biosimilars, must maintain consistent quality and clinical performance throughout their lifecycle. Drug-related authorities such as the European Medicines Agency (EMA) of the European Union, the United States Food and Drug Administration (FDA), and the Health Products and Food Branch of Health Canada hold th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicare Part D
Medicare Part D, also called the Medicare prescription drug benefit, is an optional United States federal-government program to help Medicare beneficiaries pay for self-administered prescription drugs. Part D was enacted as part of the Medicare Modernization Act of 2003 and went into effect on January 1, 2006. Under the program, drug benefits are provided by private insurance plans that receive premiums from both enrollees and the government. Part D plans typically pay most of the cost for prescriptions filled by their enrollees. However, plans are later reimbursed for much of this cost through rebates paid by manufacturers and pharmacies. Part D enrollees cover a portion of their own drug expenses by paying cost-sharing. The amount of cost-sharing an enrollee pays depends on the retail cost of the filled drug, the rules of their plan, and whether they are eligible for additional Federal income-based subsidies. Prior to 2010, enrollees were required to pay 100% of their re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |