|
GY Andromedae
GY Andromedae (GY And) is an α2 Canum Venaticorum type binary variable star in the northern constellation Andromeda. Its brightness fluctuates in visual magnitude between 6.27m and 6.41m, making it a challenge to view with the naked eye even in good seeing conditions. The magnetic activity on this star shows an unusually long period of variability, cycling about once every 23 years. Based upon parallax measurements, this star is located at a distance of about from the Earth. This is classified as an Ap/Bp star, with a peculiar spectrum showing lines of chromium and europium that change in intensity over a period matching the variability cycle, although opposite in phase. Its most striking characteristic is the presence of the unstable element promethium in its emission spectrum. All isotopes of this element are radioactive with half lives of 17.7 years or less. The promethium in the outer envelope may be generated by the spontaneous fission of higher mass transura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parallax
Parallax is a displacement or difference in the apparent position of an object viewed along two different sightline, lines of sight and is measured by the angle or half-angle of inclination between those two lines. Due to perspective (graphical), foreshortening, nearby objects show a larger parallax than farther objects, so parallax can be used to determine distances. To measure large distances, such as the distance of a planet or a star from Earth, astronomers use the principle of parallax. Here, the term ''Stellar parallax, parallax'' is the semi-angle of inclination between two sight-lines to the star, as observed when Earth is on opposite sides of the Sun in its orbit. These distances form the lowest rung of what is called "the cosmic distance ladder", the first in a succession of methods by which astronomers determine the distances to celestial objects, serving as a basis for other distance measurements in astronomy forming the higher rungs of the ladder. Because parallax ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay. Nuclear fission was discovered by chemists Otto Hahn and Fritz Strassmann and physicists Lise Meitner and Otto Robert Frisch. Hahn and Strassmann proved that a fission reaction had taken place on 19 December 1938, and Meitner and her nephew Frisch explained it theoretically in January 1939. Frisch named the process "fission" by analogy with biological fission of living cells. In their second publication on nuclear fission in February 1939, Hahn and Strassmann predicted the existence and liberation of additional neutrons during the fission process, opening up the possibility of a nuclear chain reaction. For heavy nuclides, it is an exothermic reaction which can release large amounts of energy both as electromagnetic radiat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Half-life
Half-life is a mathematical and scientific description of exponential or gradual decay. Half-life, half life or halflife may also refer to: Film * Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang * ''Half Life: A Parable for the Nuclear Age'', a 1985 Australian documentary film Literature * Half Life (Jackson novel), ''Half Life'' (Jackson novel), a 2006 novel by Shelley Jackson * Half-Life (Krach novel), ''Half-Life'' (Krach novel), a 2004 novel by Aaron Krach * Halflife (Michalowski novel), ''Halflife'' (Michalowski novel), a 2004 novel by Mark Michalowski * ''Rozpad połowiczny'' (), a 1988 award-winning dystopia novel by Edmund Wnuk-Lipiński Music *Half Life (3 album), ''Half Life'' (3 album) (2001) *Halflife (EP), ''Halflife'' (EP), an EP by Lacuna Coil and the title track *''Half-Life E.P.'', an EP by Local H * "Half Life", a song by 10 Years from ''The Autumn Effect'' * "Half Life", a song by Come from ''Near-Life Experience'' * "Ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emission Spectrum
The emission spectrum of a chemical element or chemical compound is the Spectrum (physical sciences), spectrum of frequencies of electromagnetic radiation emitted due to electrons making a atomic electron transition, transition from a high energy state to a lower energy state. The photon energy of the emitted photons is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances. Emission In physics, emission is the process by which a higher energy quantum mechanical state of a particle becomes converted to a lower one through the emis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Promethium
Promethium is a chemical element; it has Symbol (chemistry), symbol Pm and atomic number 61. All of its isotopes are Radioactive decay, radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust at any given time. Promethium is one of the only two radioactive elements that are both preceded and followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3. In 1902 Bohuslav Brauner suggested that there was a then-unknown element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley, who, having measured the atomic numbers of all the elements then known, found that the element with atomic number 61 was missing. In 1926, two groups (one Italian and one American) claimed to have isolated a sample of element 61; both "discoverie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
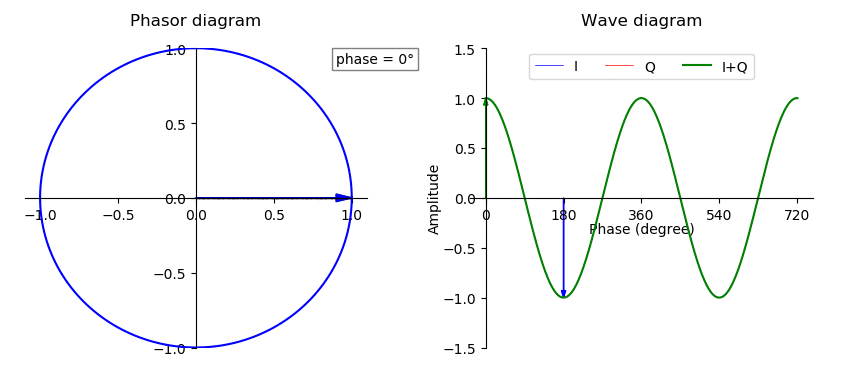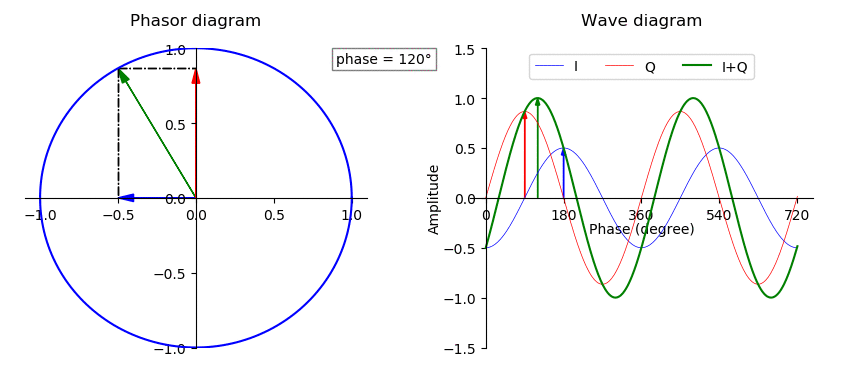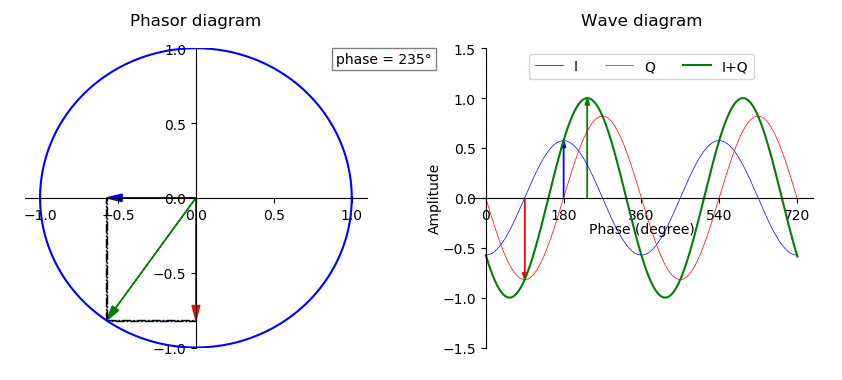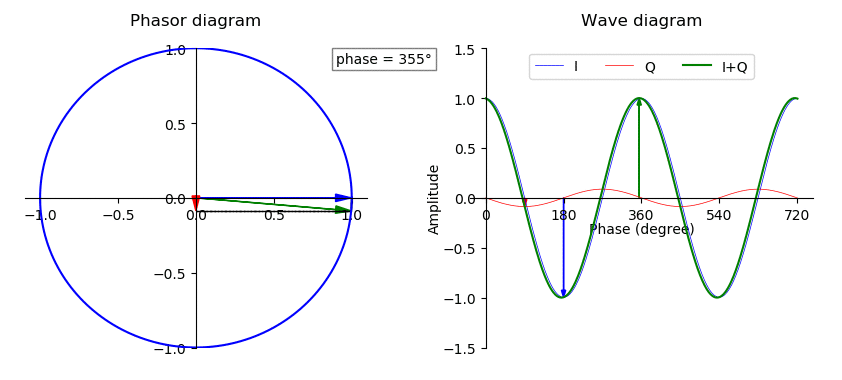
Phase (waves)
In physics and mathematics, the phase (symbol φ or ϕ) of a wave or other periodic function F of some real variable t (such as time) is an angle-like quantity representing the fraction of the cycle covered up to t. It is expressed in such a scale that it varies by one full turn as the variable t goes through each period (and F(t) goes through each complete cycle). It may be measured in any angular unit such as degrees or radians, thus increasing by 360° or 2\pi as the variable t completes a full period. This convention is especially appropriate for a sinusoidal function, since its value at any argument t then can be expressed as \varphi(t), the sine of the phase, multiplied by some factor (the amplitude of the sinusoid). (The cosine may be used instead of sine, depending on where one considers each period to start.) Usually, whole turns are ignored when expressing the phase; so that \varphi(t) is also a periodic function, with the same period as F, that repeatedly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and softest of the lanthanides. It is soft enough to be cut with a knife. Europium was discovered in 1896, provisionally designated as Σ; in 1901, it was named after the continent of Europe. Europium usually assumes the oxidation state +3, like other members of the lanthanide series, but compounds having oxidation state +2 are also common. All europium compounds with oxidation state +2 are slightly reducing. Europium has no significant biological role and is relatively non-toxic compared to other heavy metals. Most applications of europium exploit the phosphorescence of europium compounds. Europium is one of the rarest of the rare-earth elements on Earth.Stwertka, Albert. ''A Guide to the Elements'', Oxford University Press, 1996, p. 156. Ety ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal. Chromium is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polishing, polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared, infrared light. The name of the element is derived from the Ancient Greek, Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Indust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectral Line
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission (electromagnetic radiation), emission or absorption (electromagnetic radiation), absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible. Types of line spectra Spectral lines are the result of interaction between a Quantum mechanics, quantum system (usually atoms, but sometimes molecules or atomic nucleus, atomic nuclei) and a single photon. When a photon has about the right amount of photon energy, energy (which is connected to its frequency) to allow a change in the energy state of the system (in the case of an atom this is usually an electron cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peculiar Star
In astrophysics, chemically peculiar stars (CP stars) are stars with distinctly unusual metal abundances, at least in their surface layers. Classification Chemically peculiar stars are common among hot main-sequence (hydrogen-burning) stars. These hot peculiar stars have been divided into four main classes on the basis of their spectra, although two classification systems are sometimes used: * non-magnetic metallic-lined (Am, CP1) * magnetic (Ap, CP2) * non-magnetic mercury-manganese (HgMn, CP3) * helium-weak (He-weak, CP4). The class names provide a good idea of the peculiarities that set them apart from other stars on or near the main sequence. The Am stars (CP1 stars) show weak lines of singly ionized Ca and/or Sc, but show enhanced abundances of heavy metals. They also tend to be slow rotators and have an effective temperature between 7000 and . The Ap stars (CP2 stars) are characterized by strong magnetic fields, enhanced abundances of elements such as Si, Cr, Sr and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |