|
G-proteins
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of ''alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein-coupled Receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) license. Ligands can bind either to extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site within transmembrane helices (Rhodopsin-like family). They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed. G protein-coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martin Rodbell
Martin Rodbell (December 1, 1925 – December 7, 1998) was an American biochemist and molecular endocrinologist who is best known for his discovery of G-proteins. He shared the 1994 Nobel Prize in Physiology or Medicine with Alfred G. Gilman for "their discovery of G-proteins and the role of these proteins in signal transduction in cells." Biography Rodbell was born in Baltimore, Maryland, the son of Shirley (née Abrams) and Milton Rodbell, a grocer. His family was Jewish. After graduating from the Baltimore City College high school, he entered Johns Hopkins University in 1943, with interests in biology and French existential literature. In 1944, his studies were interrupted by his military service as a U.S. Navy radio operator during World War II. He returned to Hopkins in 1946 and received his B.S. in biology in 1949. In 1950, he married Barbara Charlotte Ledermann, a former friend of Margot Frank, diarist Anne Frank's older sister. Martin and Barbara had four childr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
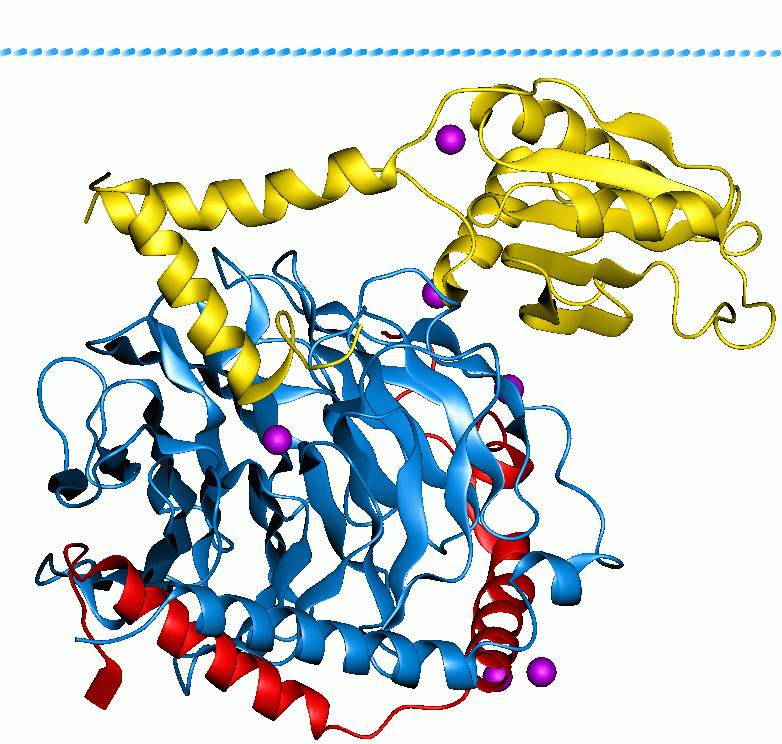
Heterotrimeric G Protein
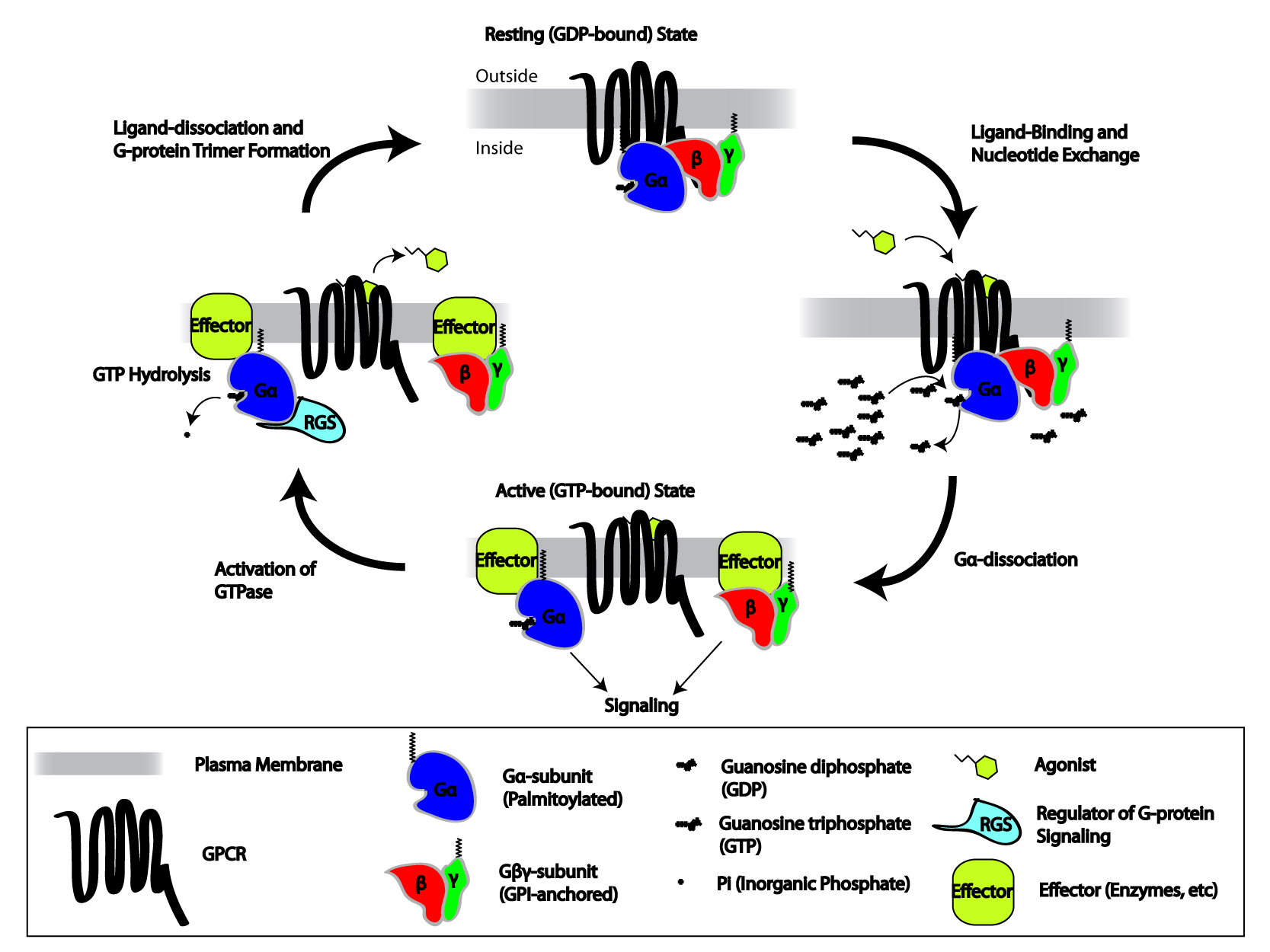
Heterotrimeric G protein, also sometimes referred to as the ''"large" G proteins'' (as opposed to the subclass of smaller, monomeric small GTPases) are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of ''alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein. When ligands bind a GPCR, the GPCR acquires GEF ( guanine nucleotide exchange factor) ability, which activates the G-protein by exchanging the GDP on the ''alpha'' subunit to GTP. The binding of GTP to the ''alpha'' subunit results in a structural change and its dissociation from the rest of the G-protein. Generally, the ''alpha'' subunit binds membrane-boun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Beta-gamma Complex
The G beta-gamma complex (Gβγ) is a tightly bound dimeric protein complex, composed of one Gβ and one Gγ subunit, and is a component of heterotrimeric G proteins. Heterotrimeric G proteins, also called guanosine nucleotide-binding proteins, consist of three subunits, called alpha, beta, and gamma subunits, or Gα, Gβ, and Gγ. When a G protein-coupled receptor (GPCR) is activated, Gα dissociates from Gβγ, allowing both subunits to perform their respective downstream signaling effects. One of the major functions of Gβγ is the inhibition of the Gα subunit. History The individual subunits of the G protein complex were first identified in 1980 when the regulatory component of adenylate cyclase was successfully purified, yielding three polypeptides of different molecular weights. Initially, it was thought that Gα, the largest subunit, was the major effector regulatory subunit, and that Gβγ was largely responsible for inactivating the Gα subunit and enhancing membrane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Triphosphate
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon. It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis. GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases. Uses Energy transfer GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GTPase
GTPases are a large family of hydrolase enzymes that bind to the nucleotide guanosine triphosphate (GTP) and hydrolyze it to guanosine diphosphate (GDP). The GTP binding and hydrolysis takes place in the highly conserved P-loop "G domain", a protein domain common to many GTPases. Functions GTPases function as molecular switches or timers in many fundamental cellular processes. Examples of these roles include: * Signal transduction in response to activation of cell surface receptors, including transmembrane receptors such as those mediating taste, smell and vision. * Protein biosynthesis (a.k.a. translation) at the ribosome. * Regulation of cell differentiation, proliferation, division and movement. * Translocation of proteins through membranes. * Transport of vesicles within the cell, and vesicle-mediated secretion and uptake, through GTPase control of vesicle coat assembly. GTPases are active when bound to GTP and inactive when bound to GDP. In the generalized r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small GTPase
Small GTPases (), also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases. A typical G-protein is active when bound to GTP and inactive when bound to GDP (i.e. when the GTP is hydrolyzed to GDP). The GDP can be then replaced by free GTP. Therefore, a G-protein can be switched on and off. GTP hydrolysis is accelerated by GTPase activating proteins (GAPs), while GTP exchange is catalyzed by guanine nucleotide exchange factors (GEFs). Activation of a GEF typically activates its cognate G-protein, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Transport Protein
A membrane transport protein (or simply transporter) is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral membrane proteins, integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion or active transport. The two main types of proteins involved in such transport are broadly categorized as either ''channels'' or ''carriers''. The Solute carrier family, solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are known as the transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drugs as well. Difference between channels and carriers A carrier is not open simultaneously to both th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transcription (genetics)
Transcription is the process of copying a segment of DNA into RNA. The segments of DNA transcribed into RNA molecules that can encode proteins are said to produce messenger RNA (mRNA). Other segments of DNA are copied into RNA molecules called non-coding RNAs (ncRNAs). mRNA comprises only 1–3% of total RNA samples. Less than 2% of the human genome can be transcribed into mRNA ( Human genome#Coding vs. noncoding DNA), while at least 80% of mammalian genomic DNA can be actively transcribed (in one or more types of cells), with the majority of this 80% considered to be ncRNA. Both DNA and RNA are nucleic acids, which use base pairs of nucleotides as a complementary language. During transcription, a DNA sequence is read by an RNA polymerase, which produces a complementary, antiparallel RNA strand called a primary transcript. Transcription proceeds in the following general steps: # RNA polymerase, together with one or more general transcription factors, binds to promot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Motility
Motility is the ability of an organism to move independently, using metabolic energy. Definitions Motility, the ability of an organism to move independently, using metabolic energy, can be contrasted with sessility, the state of organisms that do not possess a means of self-locomotion and are normally immobile. Motility differs from mobility, the ability of an object to be moved. The term vagility encompasses both motility and mobility; sessile organisms including plants and fungi often have vagile parts such as fruits, seeds, or spores which may be dispersed by other agents such as wind, water, or other organisms. Motility is genetically determined, but may be affected by environmental factors such as toxins. The nervous system and musculoskeletal system provide the majority of mammalian motility. In addition to animal locomotion, most animals are motile, though some are vagile, described as having passive locomotion. Many bacteria and other microorganisms, and multice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contractility
Contractility refers to the ability for self-contraction, especially of the muscles or similar active biological tissue *Contractile ring in cytokinesis *Contractile vacuole *Muscle contraction **Myocardial contractility *See contractile cell for an overview of cell types in humans. See also *motility Motility is the ability of an organism to move independently, using metabolic energy. Definitions Motility, the ability of an organism to move independently, using metabolic energy, can be contrasted with sessility, the state of organisms th ... {{SIA Cell movement ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secretion
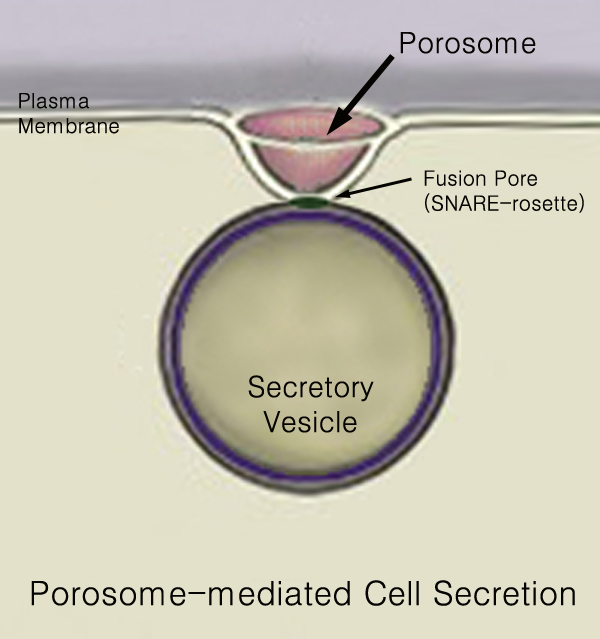
440px Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. Secretion in bacterial species means the transport or translocation of effector molecules for example: proteins, enzymes or toxins (such as cholera toxin in pathogenic bacteria e.g. '' Vibrio cholerae'') from across the interior (cytoplasm or cytosol) of a bacterial cell to its exterior. Secretion is a very important mechanism in bacterial functioning and operation in their natural surrounding environment for adaptation and survival. In eukaryotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |