|
François-Marie Raoult
François-Marie Raoult (; 10 May 1830 – 1 April 1901) was a French chemist who conducted research into the behavior of solutions, especially their physical properties. Life and work Raoult was born at Fournes, in the ''département'' of Nord. He became aspirant ''répétiteur'' at the Lycée of Reims in 1853, and after holding several intermediate positions was appointed in 1862 to the professorship of chemistry in Sens lycée. There he prepared a thesis on electromotive force which gained him a doctor's degree in Paris the following year. In 1867 Raoult was put in charge of chemistry classes at the University of Grenoble, and three years later he succeeded to the chair of chemistry, which he held until his death in 1901. Raoult's earliest researches were physical in character, being largely concerned with the phenomena of the voltaic cell; later there was a period when more purely chemical questions engaged his attention. Raoult's name is best known in connection with wor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fournes-en-Weppes
Fournes-en-Weppes () is a commune in the Nord department in northern France. It is part of the Métropole Européenne de Lille. Hitler spent nearly half of his wartime service in World War I here. Population Heraldry See also *Communes of the Nord department The following is a list of the 647 communes of the Nord department of the French Republic. The communes cooperate in the following intercommunalities (as of 2025): References Fournesenweppes French Flanders {{LilleArrondissement-geo-stub ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freezing-point Depression
Freezing-point depression is a drop in the maximum temperature at which a substance freezing, freezes, caused when a smaller amount of another, non-Volatility (chemistry), volatile substance is added. Examples include adding salt into water (used in ice cream makers and for De-icing#Chemical de-icers, de-icing roads), Alcohol (chemistry), alcohol in water, Ethylene glycol, ethylene or propylene glycol in water (used in antifreeze in cars), adding copper to molten silver (used to make Solder#Hard_solder, solder that flows at a lower temperature than the silver pieces being joined), or the mixing of two solids such as impurities into a finely powdered drug. In all cases, the substance added/present in smaller amounts is considered the solute, while the original substance present in larger quantity is thought of as the solvent. The resulting liquid solution or solid-solid mixture has a lower Melting point, freezing point than the pure solvent or solid because the chemical potentia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
19th-century French Chemists
The 19th century began on 1 January 1801 (represented by the Roman numerals MDCCCI), and ended on 31 December 1900 (MCM). It was the 9th century of the 2nd millennium. It was characterized by vast social upheaval. Slavery was Abolitionism, abolished in much of Europe and the Americas. The First Industrial Revolution, though it began in the late 18th century, expanded beyond its British homeland for the first time during the 19th century, particularly remaking the economies and societies of the Low Countries, France, the Rhineland, Northern Italy, and the Northeastern United States. A few decades later, the Second Industrial Revolution led to ever more massive urbanization and much higher levels of productivity, profit, and prosperity, a pattern that continued into the 20th century. The Catholic Church, in response to the growing influence and power of modernism, secularism and materialism, formed the First Vatican Council in the late 19th century to deal with such problems an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Légion D'Honneur
The National Order of the Legion of Honour ( ), formerly the Imperial Order of the Legion of Honour (), is the highest and most prestigious French national order of merit, both military and Civil society, civil. Currently consisting of five classes, it was originally established in 1802 by Napoleon, Napoleon Bonaparte, and it has been retained (with occasional slight alterations) by all later French governments and regimes. The order's motto is ' ("Honour and Fatherland"); its Seat (legal entity), seat is the Palais de la Légion d'Honneur next to the Musée d'Orsay, on the left bank of the Seine in Paris. Since 1 February 2023, the Order's grand chancellor has been retired General François Lecointre, who succeeded fellow retired General Benoît Puga in office. The order is divided into five degrees of increasing distinction: ' (Knight), ' (Officer), ' (Commander (order), Commander), ' (Grand Officer) and ' (Grand Cross). History Consulate During the French Revolution, all ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Society
The Chemical Society was a scientific society formed in 1841 (then named the Chemical Society of London) by 77 scientists as a result of increased interest in scientific matters. Chemist Robert Warington was the driving force behind its creation. The London Chemical Society 1824 The early days of the 1824 Chemical Society came with a rough start. Among the artisan class, the magazine ''The Chemist'', written by John Knight and Henry Lacey, had started to get some traction. Some argue that they falsely mentioned that the 1824 Chemical Society was attempting to gather an educated upper and middle-class group of chemists and philosophers. Because of this, the writers of ''The Chemist'' maintained a very practical and anti-theoretical bias, as they had lashed out at the time wasted by academic chemists researching atomic weight distributions. To find a means of how this society should be better set up and run, correspondents and proponents of ''The Chemist'' advised that membership i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ernst Otto Beckmann
Ernst Otto Beckmann (July 4, 1853 – July 12, 1923) was a German pharmacist and chemist who is remembered for his invention of the Beckmann differential thermometer and for his discovery of the Beckmann rearrangement. Scientific work Ernst Otto Beckmann was born in Solingen, Germany on July 4, 1853, to a family headed by Johannes Friedrich Wilhelm Beckmann, a manufacturer. The elder Beckmann's factory produced mineral dyes, pigments, abrasives, and polishing material, and it was there that the younger Beckmann conducted his early chemical experiments. At the age of 17, Beckmann was persuaded by his father to study pharmacy instead of chemistry, and so in 1870 an apprenticeship was arranged in Elberfeld. However, Beckmann did not enjoy the working conditions and returned home, much to his father's disappointment. After being informed that a career in chemistry would be challenging if he couldn't handle a pharmaceutical apprenticeship, Beckmann went back to Elberfeld to complete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilhelm Ostwald
Wilhelm Friedrich Ostwald (; – 4 April 1932) was a Latvian chemist and philosopher. Ostwald is credited with being one of the founders of the field of physical chemistry, with Jacobus Henricus van 't Hoff, Walther Nernst and Svante Arrhenius. He received the Nobel Prize in Chemistry in 1909 for his scientific contributions to the fields of catalysis, chemical equilibria and reaction velocities. Following his 1906 retirement from academic life, Ostwald became much involved in philosophy, art, and politics. He made significant contributions to each of these fields. He has been described as a polymath. Early life and education Ostwald was born ethnically Baltic German in Riga, Russian Empire (now Latvia) to master-cooper Gottfried Wilhelm Ostwald and Elisabeth Leuckel. He was the middle child of three, born after Eugen and before Gottfried. Ostwald developed an interest in science as a child and conducted experiments at his home, particularly related to fireworks and photogr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jacobus Henricus Van 't Hoff
Jacobus Henricus van 't Hoff Jr. (; 30 August 1852 – 1 March 1911) was a Dutch physical chemistry, physical chemist. A highly influential theoretical chemistry, theoretical chemist of his time, Van 't Hoff was the first winner of the Nobel Prize in Chemistry. His pioneering work helped found the modern theory of chemical affinity, chemical equilibrium, chemical kinetics, and chemical thermodynamics. In his 1874 pamphlet, Van 't Hoff formulated the theory of the tetrahedral carbon atom and laid the foundations of stereochemistry. In 1875, he predicted the correct structures of allenes and cumulenes as well as their axial Chirality (chemistry), chirality. He is also widely considered one of the founders of physical chemistry as the discipline is known today. Biography The third of seven children, Van 't Hoff was born in Rotterdam, Netherlands, 30 August 1852. His father was Jacobus Henricus van 't Hoff Sr., a physician, and his mother was Alida Kolff van 't Hoff. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid (or solid) in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions. The vapor p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
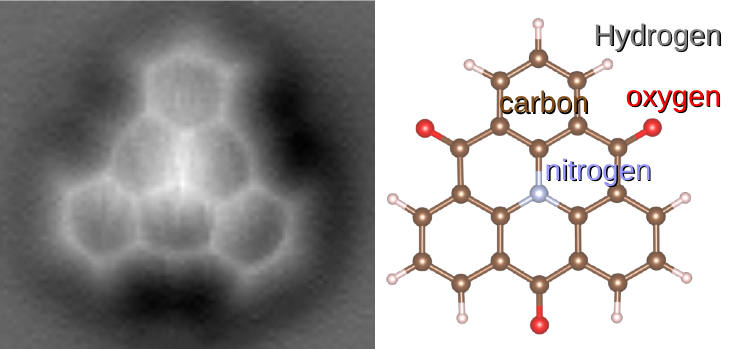
Molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilibrium, equilibrium. The melting point of a substance depends on pressure and is usually specified at a Standard temperature and pressure, standard pressure such as 1 Atmosphere (unit), atmosphere or 100 Pascal (unit), kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to Supercooling, supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the #Melting point measurements, melting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |