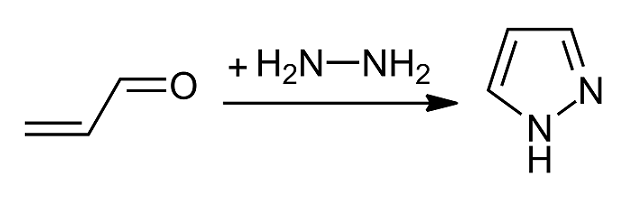|
Fomepizole
Fomepizole, also known as 4-methylpyrazole, is a medication used to treat methanol and ethylene glycol poisoning. It may be used alone or together with hemodialysis. It is given by injection into a vein. Common side effects include headache, nausea, sleepiness, and unsteadiness. It is unclear if use during pregnancy causes risk to a fetus. Fomepizole works by blocking the enzyme that converts methanol and ethylene glycol to their toxic breakdown products. Fomepizole was approved for medical use in the United States in 1997. It is on the World Health Organization's List of Essential Medicines. Medical uses Fomepizole is used to treat ethylene glycol and methanol poisoning. It acts to inhibit the conversion of these alcohols into their respective aldehydes by alcohol dehydrogenase. The prevents further conversion to the more active toxic metabolites oxalic acid and formic acid, respectively. Fomepizole is most effective when given soon after ingestion of ethylene glycol or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol Poisoning
Ethylene glycol poisoning is poisoning caused by drinking ethylene glycol. Early symptoms include intoxication, vomiting and abdominal pain. Later symptoms may include a decreased level of consciousness, headache, and seizures. Long term outcomes may include kidney failure and brain damage. Toxicity and death may occur after drinking even in a small amount as ethylene glycol is more toxic than other diols. Ethylene glycol is a colorless, odorless, sweet liquid, commonly found in antifreeze. It may be drunk accidentally or intentionally in a suicide attempt. When broken down by the body it results in glycolic acid and oxalic acid which cause most of the toxicity. The diagnosis may be suspected when calcium oxalate crystals are seen in the urine or when acidosis or an increased osmol gap is present in the blood. Diagnosis may be confirmed by measuring ethylene glycol levels in the blood; however, many hospitals do not have the ability to perform this test. Early treatment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol Poisoning
Methanol toxicity (also methanol poisoning) is poisoning from methanol, characteristically via ingestion. Symptoms may include an altered/decreased level of consciousness, poor or no coordination, vomiting, abdominal pain, and a specific smell on the breath. Decreased vision may start as early as twelve hours after exposure. Long-term outcomes may include blindness and kidney failure. Blindness may occur after drinking as little as 10 mL; death may occur after drinking quantities over 15 mL (median 100 mL, varies depending on body weight). Methanol poisoning most commonly occurs following the drinking of windshield washer fluid. This may be accidental or as part of an attempted suicide. Toxicity may also rarely occur through extensive skin exposure or breathing in fumes. When the body breaks down methanol it results in the creation of metabolite byproducts such as formaldehyde, formic acid, and formate which cause much of the toxicity. The diagnosis may be suspected when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol Dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. In humans and many other animals, they serve to break down alcohols that are otherwise toxic, and they also participate in the generation of useful aldehyde, ketone, or alcohol groups during the biosynthesis of various metabolites. In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+. Evolution Genetic evidence from comparisons of multiple organisms showed that a glutathione-dependent formaldehyde dehydrogenase, identical to a class III alcohol dehydrogenase (ADH-3/ADH5), is presumed to be the ancestral enzyme for the entire ADH family. Early on in evolution, an effective method for eliminating both endogenous and exogenous fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antidotes
An antidote is a substance that can counteract a form of poisoning. The term ultimately derives from the Greek term φάρμακον ἀντίδοτον ''(pharmakon antidoton)'', "(medicine) given as a remedy". An older term in English which is now rare is atterlothe, derived from " atter" ("poison" or "venom"). Antidotes for anticoagulants are sometimes referred to as reversal agents. The antidotes for some particular toxins are manufactured by injecting the toxin into an animal in small doses and extracting the resulting antibodies from the host animals' blood. This results in an antivenom that can be used to counteract venom produced by certain species of snakes, spiders, and other venomous animals. Some animal venoms, especially those produced by arthropods (such as certain spiders, scorpions, and bees) are only potentially lethal when they provoke allergic reactions and induce anaphylactic shock; as such, there is no "antidote" for these venoms; however anaphylactic shock ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol Dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. In humans and many other animals, they serve to break down alcohols that are otherwise toxic, and they also participate in the generation of useful aldehyde, ketone, or alcohol groups during the biosynthesis of various metabolites. In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+. Evolution Genetic evidence from comparisons of multiple organisms showed that a glutathione-dependent formaldehyde dehydrogenase, identical to a class III alcohol dehydrogenase (ADH-3/ADH5), is presumed to be the ancestral enzyme for the entire ADH family. Early on in evolution, an effective method for eliminating both endogenous and exogenous fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formic Acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol. Natural occurrence Formic acid, which has a pungent, penetrating odor, is found naturally in insects, weeds, fruits and vegetables, and forest emissions. It appears in most ants and in stingless bees of the genus '' Oxytrigona''. Wood ants from the genus ''Formica'' can spray formic acid on their prey or to defend the nest. The puss moth caterpillar (''Cerura vinula'') will spray it as well when threatened by predators. It is also found in the trichomes of stinging nettle (''Urtica dioica''). Apart from that, this acid is incorporated in many fruits such as pineapple (0.21 mg per 100 g), apple (2 mg per ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Acidosis
Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the body's acid-base balance. Metabolic acidosis has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids. Metabolic acidosis can lead to acidemia, which is defined as arterial blood pH that is lower than 7.35. Acidemia and acidosis are not mutually exclusive – pH and hydrogen ion concentrations also depend on the coexistence of other acid-base disorders; therefore, pH levels in people with metabolic acidosis can range from low to high. Acute metabolic acidosis, lasting from minutes to several days, often occurs during serious illnesses or hospitalizations, and is generally caused when the body produces an excess amount of organic acids ( ketoacids in ketoacidosis, or lactic acid in lactic acidosis). A state of chronic metabolic acidosis, lasting several weeks to years, can be the result of impaired kidney fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as aqueous solutions (formalin), which consists mainly of the hydrate CH2(OH)2. It is the simplest of the aldehydes (). As a precursor to many other materials and chemical compounds, in 2006 the global production of formaldehyde was estimated at 12 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Formaldehyde also occurs naturally. It is derived from the degradation of serine, dimethylglycine, and lipids. Demethylases act by converting N-methyl groups to formaldehyde. Formaldehyde is classified as a group 1 carcinogen and can cause respiratory and skin irritation upon exposure. Forms Formaldehyde is more complicated than many simple carbon compounds in that i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde Dehydrogenase
In enzymology, a formaldehyde dehydrogenase () is an enzyme that catalyzes the chemical reaction :formaldehyde + NAD+ + H2O \rightleftharpoons formate + NADH + H+ The 3 substrates of this enzyme are formaldehyde, NAD+, and H2O, whereas its 3 products are formate, NADH, and H+. This enzyme belongs to the family of oxidoreductases, specifically those acting on the aldehyde or oxo group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is formaldehyde:NAD+ oxidoreductase. Other names in common use include NAD+-linked formaldehyde dehydrogenase, s-nitrosoglutathione reductase (GSNO reductase) and NAD+-dependent formaldehyde dehydrogenase. This enzyme participates in methane metabolism. Ubiquitous function S-nitrosoglutathione reductase (GSNOR) is a class III alcohol dehydrogenase (ADH) encoded by the ADH5 gene in humans. It is a primordial ADH that is ubiquitously expressed in plant and animals alike. GSNOR reduces S-nitrosoglutathione (GSN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water per os, by mouth. It may also be used to administer pharmaceutical drug, medications or other medical therapy such as blood transfusion, blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatic
The liver is a major metabolic organ (anatomy), organ exclusively found in vertebrates, which performs many essential biological Function (biology), functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of various proteins and various other Biochemistry, biochemicals necessary for digestion and growth. In humans, it is located in the quadrants and regions of abdomen, right upper quadrant of the abdomen, below the thoracic diaphragm, diaphragm and mostly shielded by the lower right rib cage. Its other metabolic roles include carbohydrate metabolism, the production of a number of hormones, conversion and storage of nutrients such as glucose and glycogen, and the decomposition of red blood cells. Anatomical and medical terminology often use the prefix List of medical roots, suffixes and prefixes#H, ''hepat-'' from ἡπατο-, from the Greek language, Greek word for liver, such as hepatology, and hepatitis The liver is also an accessory digestive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazoles
Pyrazole is an organic compound with the formula . It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Properties Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å History The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883. In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and diazomethane. Preparation Pyrazoles are syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |