|
Fluorocyclohexane
Fluorocyclohexane is an organofluorine compound with the chemical formula . Synthesis Fluorocyclohexane is prepared by reaction of cyclohexanol with hydrogen fluoride. Safety The compound causes serious skin and eye irritation, and may also cause respiratory irritation. See also * Fluoroalkanes * Fluorocyclopropane * Fluorobenzene Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated Phenyl group, PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds. Preparation PhF was first reported in 18 ... References {{Fluorine compounds Cyclohexanes Fluorine compounds Organofluorides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorocyclohexane
Cyclohexyl chloride (or chlorocyclohexane) is a chlorinated hydrocarbon with the formula (CH2)5CHCl. It is a colorless liquid. Cyclohexyl chloride can be prepared from cyclohexanol by treatment with hydrogen chloride The Chemical compound, compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hyd .... References Organochlorides Cyclohexyl compounds Cyclohexanes {{organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromocyclohexane
Bromocyclohexane (also called cyclohexyl bromide, abbreviated CXB) is an organic compound with the chemical formula . Uses and reactions It is used to match the refractive index of PMMA for example in confocal microscopy of colloids. A mixture of ''cis''-decalin and CXB can simultaneously match optical index and density of PMMA. Due to the moderate dielectric constant of CXB (ε = 7.9 ), PMMA acquires charges that can be screened by the addition of salt (e.g. tetrabutyl ammonium bromide), leading to a very good approximation of colloidal hard sphere. A drawback is that CXB is a good solvent for PMMA, causing it to swell over time, which may lead to a poor determination of particle radii and determination of solid volume fraction. It is a standard coupling partner of cross coupling reactions. Similarly, cyclohexyl bromide is a standard alkylating agent. Stated applications in drug chemistry include the synthesis of gamfexine, drofenine, trihexyphenidyl, procyclidine, feclem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodocyclohexane
Iodocyclohexane is an organoiodine compound with the chemical formula . Synthesis Iodocyclohexane has been prepared by the addition of hydrogen iodide to cyclohexene. Alternatively, it can be prepared by the reaction of cyclohexane and iodoform. Physical properties Iodocyclohexane forms colorless to slightly reddish yellow liquid. It is soluble in ethanol, ether, and acetone. Uses The compound has been used as reagent in demethylation of aryl methyl ethers in DMF under reflux condition. See also * Iodoalkanes * Iodobenzene Iodobenzene is an aryl iodide and the simplest of the iodobenzenes, consisting of a benzene ring substituted with one iodine atom. Its chemical formula is . It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorles ... References {{Iodine compounds Cyclohexanes Iodine compounds Organoiodides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocyclopropane
Fluorocyclopropane is an organofluorine compound with the chemical formula . The compound is a member of haloalkane family. Synthesis The compound can be produced by reacting imidazolylidene cyclopropyl group with xenon difluoride. Also, a reaction of enantioselective cyclopropanation of fluoro-substituted allylic alcohols using zinc carbenoids. See also * Fluoroalkane Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, ...s * Fluorocyclohexane References Extra reading * * {{Fluorine compounds Cyclopropyl compounds Fluorine compounds Organofluorides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine Compound
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/mol). This is significantly st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fisher Scientific
Fisher Scientific International, Inc. (NYSE: FSH) was a laboratory supply and biotechnology company that provided products and services to the global scientific research and clinical laboratory markets until its merger with Thermo Electron in 2006, after which it became Thermo Fisher Scientific. The company offered products and services to over 350,000 customers located in approximately 150 countries including pharmaceutical and biotechnology companies, secondary and higher education institutions, hospitals and medical research institutions, and quality control, process control and research and development laboratories. History The company was founded in Pittsburgh, Pennsylvania, in 1902 by Chester Garfield Fisher (1881–1965), originally called the "Scientific Materials Co.". After obtaining his degree in engineering at Western University of Pennsylvania (now University of Pittsburgh), C.G. Fisher purchased the stockroom of the Pittsburgh Testing Laboratory. Fisher became a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Press
The CRC Press, LLC is an American publishing group that specializes in producing technical books. Many of their books relate to engineering, science and mathematics. Their scope also includes books on business, forensics and information technology. CRC Press is now a division of Taylor & Francis, itself a subsidiary of Informa. History The CRC Press was founded as the Chemical Rubber Company (CRC) in 1903 by brothers Arthur, Leo and Emanuel Friedman in Cleveland, Ohio, based on an earlier enterprise by Arthur, who had begun selling rubber laboratory aprons in 1900. The company gradually expanded to include sales of laboratory equipment to chemist A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...s. In 1913 the CRC offered a short (116-page) manual called the ''Rubber Handboo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanol
Cyclohexanol is the organic compound with the formula HOCH(CH2)5. The molecule is related to cyclohexane by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colorless solid with a camphor-like odor, which, when very pure, melts near room temperature. Millions of tonnes are produced annually, mainly as a precursor to nylon.Michael Tuttle Musser "Cyclohexanol and Cyclohexanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Production Cyclohexanol is produced by the oxidation of cyclohexane in air, typically using cobalt catalysts: :2 C6H12 + O2 → 2 C6H11OH This process coforms cyclohexanone, and this mixture ("KA oil" for ketone-alcohol oil) is the main feedstock for the production of adipic acid. The oxidation involves radicals and the intermediacy of the hydroperoxide C6H11O2H. Alternatively, cyclohexanol can be produced by the hydrogenation of phenol: :C6H5OH + 3 H2 → C6H11OH This process can al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
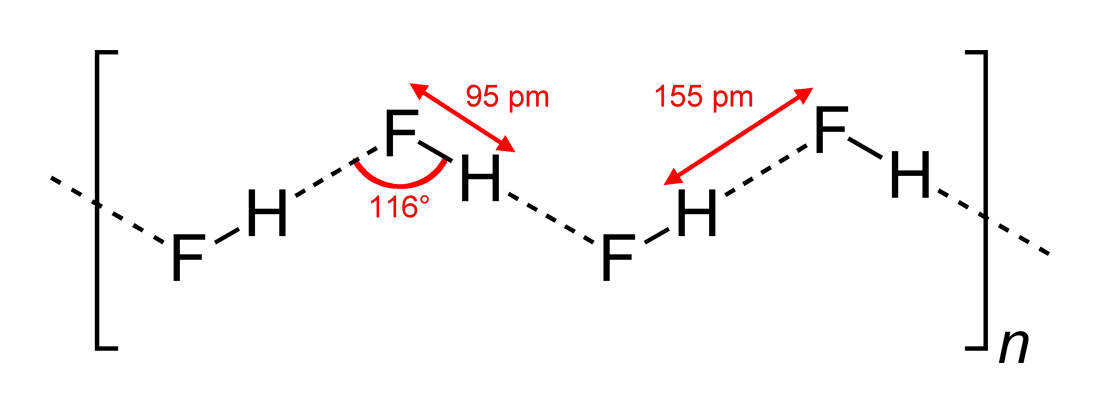
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroalkane
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/mol). This is significantly st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorobenzene
Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated Phenyl group, PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds. Preparation PhF was first reported in 1886 by O. Wallach at the University of Bonn, who prepared the compound in two steps. Phenyldiazonium chloride was first converted to a triazene using piperidine: :[PhN2]Cl + 2 (CH2)5NH → PhN=N-N(CH2)5 + [(CH2)5NH2]Cl The triazine was then cleaved with hydrofluoric acid: :PhN=N-N(CH2)5 + 2 HF → PhF + N2 + [(CH2)5NH2]F Historical note: in Wallach's era, the element fluorine was symbolized with "Fl". Thus, his procedure is subtitled "Fluorbenzol, C6H5Fl". On the laboratory scale, PhF is prepared by the thermal decomposition of the benzenediazonium tetrafluoroborate: :PhN2BF4 → PhF + BF3 + N2 According to the procedure, solid [PhN2]BF4 is heated with a flame to initiate an exothermic reaction, which also affords bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexanes
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexane is mainly used for the industrial production of adipic acid and caprolactam, which are precursors to nylon. Cyclohexyl () is the alkyl substituent of cyclohexane and is abbreviated Cy. Production Cyclohexane is one of components of naphtha, from which it can be extracted by advanced distillation methods. Distillation is usually combined with isomerization of methylcyclopentane, a similar component extracted from naphtha by similar methods. Together, these processes cover only a minority (15-20%) of the modern industrial demand, and are complemented by synthesis. Modern industrial synthesis On an industrial scale, cyclohexane is produced by hydrogenation of benzene in the presence of a Raney nickel catalyst. Producers of cyclohexane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |