|
Fluoro
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern prod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, i.e., they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its strength is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally discovered the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high- molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. Where used as a lubri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorochemical Industry
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the second largest. Asia Pacific is the fastest growing region of production. China in particular has experienced significant growth as a fluorochemical market and is becoming a producer of them as well. Fluorite mining (the main source of fluorine) was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year. Mined fluorite is separated into two main grades, with about equal production of each. ''Acidspar'' is at least 97% CaF2; ''metspar'' is much lower purity, 60–85%. (A small amount of the intermediate, ''ceramic'', grade is also made.) Metspar is used almost exclusively for iron smelting. Acidspar is primarily converted to hydrofluoric acid (by reaction with sulfuric acid). The resultant HF is mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refrigerant
A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated due to their toxicity, flammability and the contribution of CFC and HCFC refrigerants to ozone depletion and that of HFC refrigerants to climate change. Refrigerants are used in a Direct Expansion (DX) system to transfer energy from one environment to another, typically from inside a building to outside (or vice versa) commonly known as an "air conditioner" or "heat pump". Refrigerants can carry per kg 10 times more energy than water and 50 times more than air. Refrigerants are controlled substances due to 1) High Pressures (100-145 psi), 2) Extreme temperatures (-50°C to 145°C), 3) Flammability A1 class non-flammable, A2/A2L class flammable & A3 class extremely flammable/explosive and 4) Toxicity B1-low, B2-medium & B3-high, as cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Fluoridation
Water fluoridation is the controlled adjustment of fluoride to a public water supply solely to reduce tooth decay. Fluoridated water contains fluoride at a level that is effective for preventing cavities; this can occur naturally or by adding fluoride. Fluoridated water operates on tooth surfaces: in the mouth, it creates low levels of fluoride in saliva, which reduces the rate at which tooth enamel demineralizes and increases the rate at which it remineralizes in the early stages of cavities. Typically a fluoridated compound is added to drinking water, a process that in the U.S. costs an average of about $ per person-year. Defluoridation is needed when the naturally occurring fluoride level exceeds recommended limits. In 2011, the World Health Organization suggested a level of fluoride from 0.5 to 1.5 mg/L (milligrams per litre), depending on climate, local environment, and other sources of fluoride. Bottled water typically has unknown fluoride levels. Tooth decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon–fluorine Bond
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry (after the B–F single bond, Si–F single bond, and H–F single bond), and relatively short, due to its partial ionic character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound. As such, fluoroalkanes like tetrafluoromethane (carbon tetrafluoride) are some of the most unreactive organic compounds. Electronegativity and bond strength The high electronegativity of fluorine (4.0 for fluorine vs. 2.5 for carbon) gives the carbon–fluorine bond a significant polarity or dipole moment. The electron density is concentrated around the fluorine, leaving the carbon relatively electron poor. This introduces ionic character to the bond through partial charges (Cδ+—Fδ−). The partial charges on the fluorine and carbon are attract ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse Gas
A greenhouse gas (GHG or GhG) is a gas that absorbs and emits radiant energy within the thermal infrared range, causing the greenhouse effect. The primary greenhouse gases in Earth's atmosphere are water vapor (), carbon dioxide (), methane (), nitrous oxide (), and ozone (). Without greenhouse gases, the average temperature of Earth's surface would be about , rather than the present average of . The atmospheres of Venus, Mars and Titan also contain greenhouse gases. Human activities since the beginning of the Industrial Revolution (around 1750) have increased the atmospheric concentration of carbon dioxide by over 50%, from 280 ppm in 1750 to 421 ppm in 2022. The last time the atmospheric concentration of carbon dioxide was this high was over 3 million years ago. This increase has occurred despite the absorption of more than half of the emissions by various natural carbon sinks in the carbon cycle. At current greenhouse gas emission rates, temperatures co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with disco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
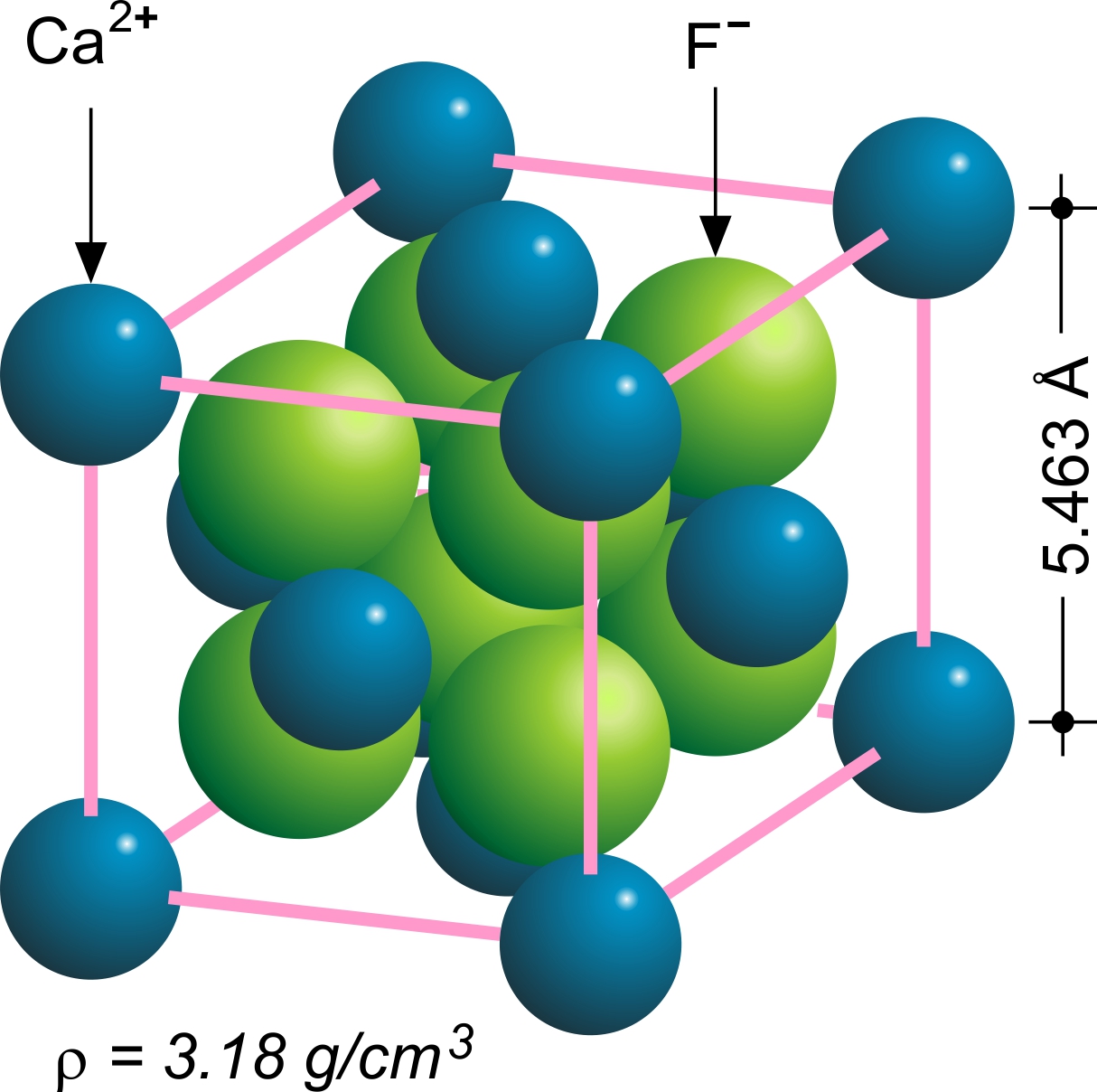
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic. Hist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hall–Héroult Process
The Hall–Héroult process is the major industrial process for smelting aluminium. It involves dissolving aluminium oxide (alumina) (obtained most often from bauxite, aluminium's chief ore, through the Bayer process) in molten cryolite, and electrolyzing the molten salt bath, typically in a purpose-built cell. The Hall–Héroult process applied at industrial scale happens at 940–980 °C and produces 99.5–99.8% pure aluminium. Recycled aluminum requires no electrolysis, thus it does not end up in this process. This process contributes to climate change through the emission of carbon dioxide and fluorocarbons in the electrolytic reaction and consumption of large amounts of electrical energy. Process Difficulties faced Elemental aluminium cannot be produced by the electrolysis of an aqueous aluminium salt, because hydronium ions readily oxidize elemental aluminium. Although a molten aluminium salt could be used instead, aluminium oxide has a melting point of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |