|
Evinacumab
Evinacumab, sold under the brand name Evkeeza, is a monoclonal antibody medication for the treatment of homozygous familial hypercholesterolemia (HoFH). Common side effects include nasopharyngitis (cold), influenza-like illness, dizziness, rhinorrhea (runny nose), and nausea. Serious hypersensitivity (allergic) reactions have occurred in the Evkeeza clinical trials. Evinacumab binds to the angiopoietin-like protein 3 (ANGPTL3). ANGPTL3 slows the function of certain enzymes that break down fats in the body. Evinacumab blocks ANGPTL3, allowing faster break down of fats that lead to high cholesterol. Evinacumab was approved for medical use in the United States in February 2021. The U.S. Food and Drug Administration considers it to be a first-in-class medication. History Regeneron invented evinacumab. The effectiveness and safety of evinacumab were evaluated in a double-blind, randomized, placebo-controlled, 24-week trial enrolling 65 participants with homozygous familial hyp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Familial Hypercholesterolemia
Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels of low-density lipoprotein cholesterol (LDL cholesterol), in the blood and early cardiovascular diseases. The most common mutations diminish the number of functional LDL receptors in the liver or produce abnormal LDL receptors that never go to the cell surface to function properly (abnormal trafficking). Since the underlying body biochemistry is slightly different in individuals with FH, their high cholesterol levels are less responsive to the kinds of cholesterol control methods which are usually more effective in people without FH (such as dietary modification and statin tablets). Nevertheless, treatment (including higher statin doses and PCSK9 inhibitors) is usually effective. FH is classified as a type 2 familial dyslipidemia. There are five types of familial dyslipidemia (not including subtypes), and each are classified from both the altered l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
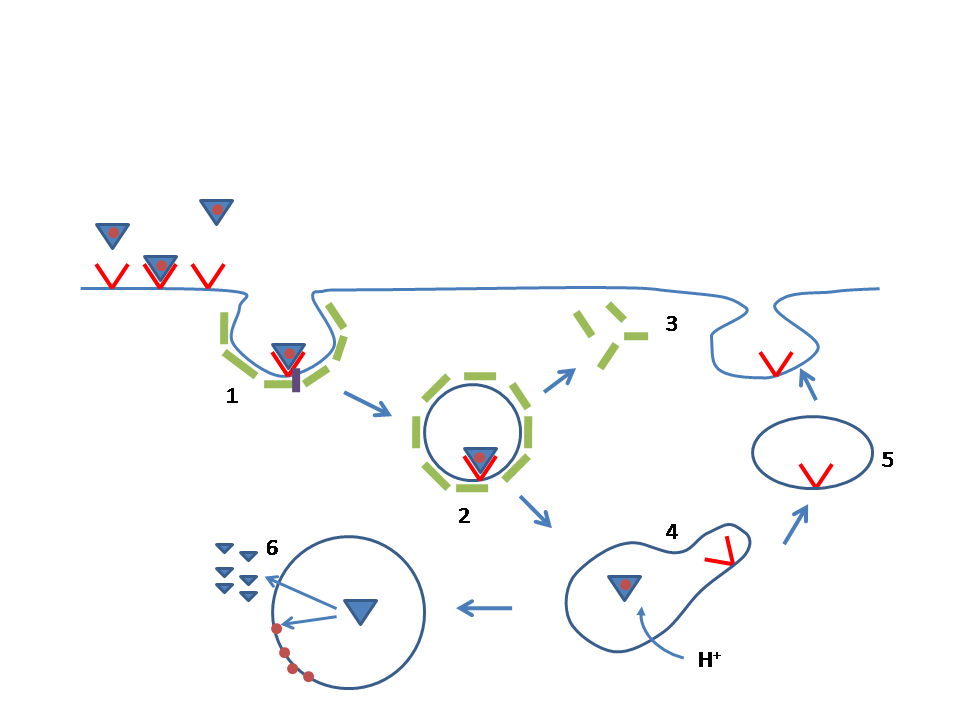
ANGPTL3
Angiopoietin-like 3, also known as ANGPTL3, is a protein that in humans is encoded by the ''ANGPTL3'' gene. Function The protein encoded by this gene is a member of the angiopoietin-like family of secreted factors. It is expressed predominantly in the liver, and has the characteristic structure of angiopoietins, consisting of a signal peptide, N-terminal coiled-coil domain, and the C-terminal fibrinogen (FBN)-like domain. The FBN-like domain in angiopoietin-like 3 protein was shown to bind alpha-5/beta-3 integrins, and this binding induced endothelial cell adhesion and migration. This protein may also play a role in the regulation of angiogenesis. Angptl3 also acts as dual inhibitor of lipoprotein lipase (LPL) and endothelial lipase (EL), thereby increasing plasma triglyceride, LDL cholesterol and HDL cholesterol in mice and humans. ANGPTL3 inhibits endothelial lipase hydrolysis Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water per os, by mouth. It may also be used to administer pharmaceutical drug, medications or other medical therapy such as blood transfusion, blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Canada
Health Canada (HC; )Health Canada is the applied title under the Federal Identity Program; the legal title is Department of Health (). is the Structure of the Canadian federal government#Departments, with subsidiary units, department of the Government of Canada responsible for national health policy. The department itself is also responsible for numerous federal health-related agencies, including the Canadian Food Inspection Agency (CFIA) and the Public Health Agency of Canada (PHAC), among others. These organizations help to ensure compliance with federal law in a variety of Healthcare in Canada, healthcare, Agriculture in Canada, agricultural, and Pharmaceutics, pharmaceutical activities. This responsibility also involves extensive collaboration with various other federal- and provincial-level organizations in order to ensure the safety of food, health, and Medication, pharmaceutical products—including the regulation of health research and pharmaceutical manufacturing/Clinical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclonal Antibody
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell. Monoclonal antibodies are identical and can thus have monovalent affinity, binding only to a particular epitope (the part of an antigen that is recognized by the antibody). In contrast, polyclonal antibodies are mixtures of antibodies derived from multiple plasma cell lineages which each bind to their particular target epitope. Artificial antibodies known as bispecific monoclonal antibodies can also be engineered which include two different antigen binding sites ( FABs) on the same antibody. It is possible to produce monoclonal antibodies that specifically bind to almost any suitable substance; they can then serve to detect or purify it. This capability has become an investigative tool in biochemistry, molecular biology, and medicine. Monoclonal antibod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medication
Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to medical diagnosis, diagnose, cure, treat, or preventive medicine, prevent disease. Drug therapy (pharmacotherapy) is an important part of the medicine, medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are Drug class, classified in many ways. One of the key divisions is by level of controlled substance, control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the medical prescription) from over-the-counter drugs (those that consumers can order for themselves). Medicines may be classified by mode of action, route of administration, biological system affected, or therapeutic effects. The World Health Organization keeps a list of essential medicines. Drug discovery and drug development are complex and expensive endeavors undertake ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First-in-class Medication
A first-in-class medication is a prototype drug that uses a "new and unique mechanism of action" to treat a particular medical condition. While the Food and Drug Administration's Center for Drug Evaluation and Research tracks first-in-class medications and reports on them annually, first-in-class is not considered a regulatory category. Although many first-in-class medications qualify as breakthrough therapies, Regenerative Medicine Advanced Therapies and/or orphan drugs, first-in-class status itself has no regulatory effect. Examples Controversy Safety By definition, a first-in-class drug does not have the safety evidence from analogous products that not-first-in-class drugs would have. However, a study investigating recalls and warnings in relation to first-in-class drugs approved between 1997 and 2012 by Health Canada has found that first-in-class drugs actually have a more favourable benefit-to-harm ratio. Economics First-in-class drugs are often seen as commerc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regeneron Pharmaceuticals
Regeneron Pharmaceuticals, Inc. is an American biotechnology company headquartered in Westchester County, New York. The company was founded in 1988. Originally focused on neurotrophic factors and their regenerative capabilities, giving rise to its present name; the company has since expanded operations into the study of both cytokine and tyrosine kinase receptors, which gave rise to their first product, which is a VEGF-trap. Company history The company was founded by CEO Leonard Schleifer and scientist George Yancopoulos in 1988. Regeneron has developed aflibercept, a VEGF inhibitor, and rilonacept, an interleukin-1 blocker. VEGF is a protein that normally stimulates the growth of blood vessels, and interleukin-1 is a protein that is normally involved in inflammation. On March 26, 2012, Bloomberg announced that Sanofi and Regeneron were in development of a new drug that would help reduce cholesterol up to 72% more than its competitors. The new drug would target the PCS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low-density Lipoprotein
Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons (aka ULDL by the overall density naming convention), very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to Cell (biology), cells. LDL has been associated with the progression of atherosclerosis. Overview Lipoproteins transfer lipids (fats) around the body in the extracellular fluid, making fats available to body cells for receptor-mediated endocytosis. Lipoproteins are complex particles composed of multiple proteins, typically 80–100 proteins per particle (organized by a single apolipoprotein B for LDL and the larger particles). A single LDL particle is about 22–27.5 nanometers in diameter, typically transporting 3,000 to 6,000 fat molecules per part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a medication, pharmaceutical agent that is developed to treat certain rare medical conditions. An orphan drug would not be profitable to produce without government assistance, due to the small population of patients affected by the conditions. The conditions that orphan drugs are used to treat are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy that depends on the legislation (if there is any) of the country. Designation of a drug as an orphan drug has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug medical research, research and development. Examples of this can be that in the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Breakthrough Therapy
Breakthrough therapy is a United States Food and Drug Administration designation that expedites drug development that was created by Congress under Section 902 of the 9 July 2012 Food and Drug Administration Safety and Innovation Act. The FDA's "breakthrough therapy" designation is not intended to imply that a drug is actually a "breakthrough" or that there is high-quality evidence of treatment efficacy for a particular condition; rather, it allows the FDA to grant priority review to drug candidates if preliminary clinical trials indicate that the therapy may offer substantial treatment advantages over existing options for patients with serious or life-threatening diseases. The FDA has other mechanisms for expediting the review and approval process for promising drugs, including fast track designation, accelerated approval, and priority review. Requirements A breakthrough therapy designation can be assigned to a drug if "it is a drug which is intended alone or in combination wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |