|
European Medicines Agency (EMA)
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been described as a ''sui generis'' political entity (without precedent or comparison) combining the characteristics of both a federation and a confederation. Containing 5.8per cent of the world population in 2020, the EU generated a nominal gross domestic product (GDP) of around trillion in 2021, constituting approximately 18per cent of global nominal GDP. Additionally, all EU states but Bulgaria have a very high Human Development Index according to the United Nations Development Programme. Its cornerstone, the Customs Union, paved the way to establishing an internal single market based on standardised legal framework and legislation that applies in all member states in those matters, and only those matters, where the states have agree ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Parliament
The European Parliament (EP) is one of the legislative bodies of the European Union and one of its seven institutions. Together with the Council of the European Union (known as the Council and informally as the Council of Ministers), it adopts European legislation, following a proposal by the European Commission. The Parliament is composed of 705 members (MEPs). It represents the second-largest democratic electorate in the world (after the Parliament of India), with an electorate of 375 million eligible voters in 2009. Since 1979, the Parliament has been directly elected every five years by the citizens of the European Union through universal suffrage. Voter turnout in parliamentary elections decreased each time after 1979 until 2019, when voter turnout increased by eight percentage points, and rose above 50% for the first time since 1994. The voting age is 18 in all EU member states except for Malta and Austria, where it is 16, and Greece, where it is 17. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Union
The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The union has a total area of and an estimated total population of about 447million. The EU has often been described as a ''sui generis'' political entity (without precedent or comparison) combining the characteristics of both a federation and a confederation. Containing 5.8per cent of the world population in 2020, the EU generated a nominal gross domestic product (GDP) of around trillion in 2021, constituting approximately 18per cent of global nominal GDP. Additionally, all EU states but Bulgaria have a very high Human Development Index according to the United Nations Development Programme. Its cornerstone, the Customs Union, paved the way to establishing an internal single market based on standardised legal framework and legislation that applies in all member states in those matters, and only those matters, where the states have agree ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacovigilance
Term Given By Tushar Sharma (UPES Batch 2025) Pharmacovigilance (PV, or PhV), also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: (Greek for drug) and (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy (the condition that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function was excluded with the latest amendment of the applicable legislation). Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
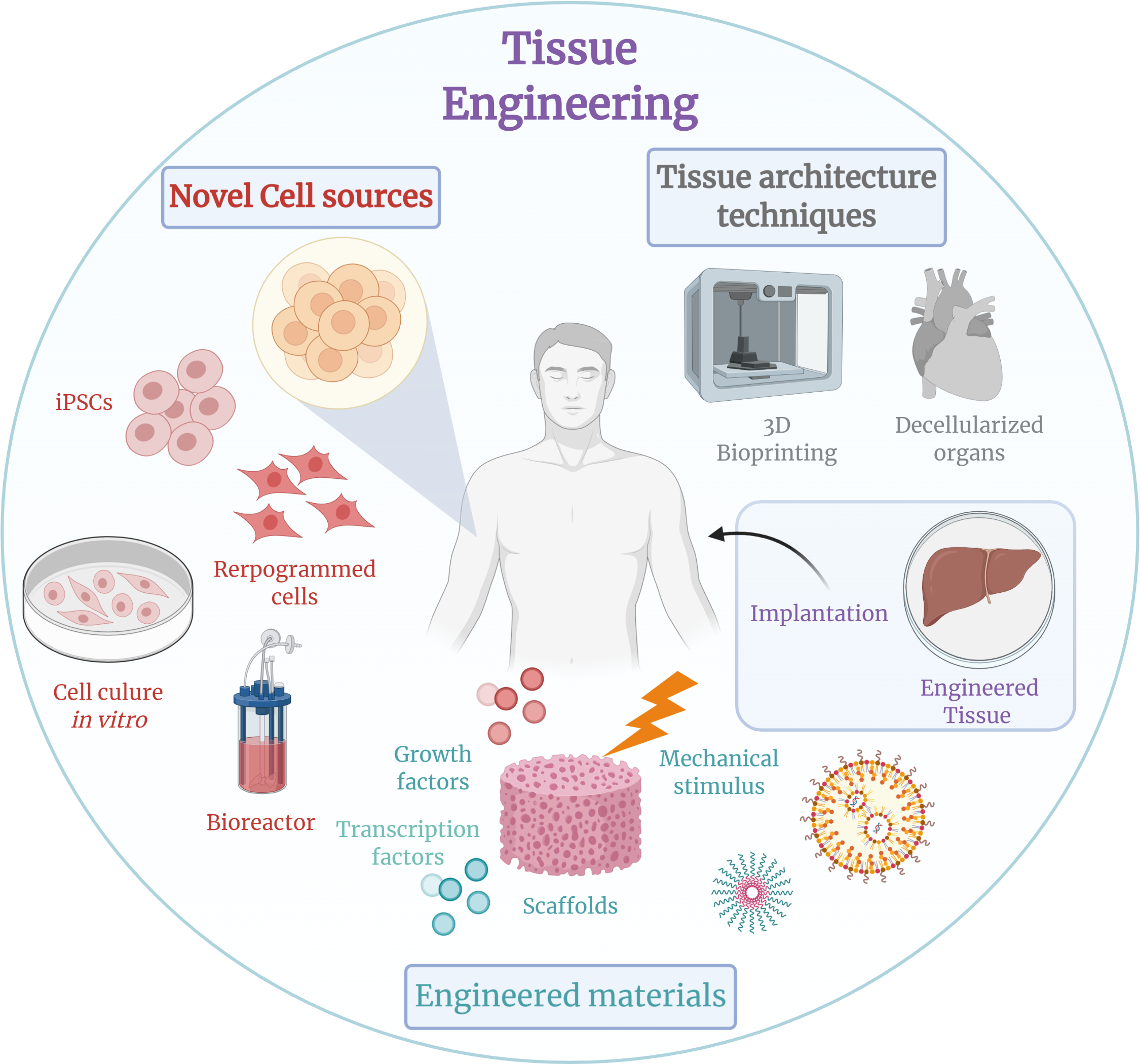
Tissue Engineering
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field of its own. While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e. bone, cartilage, blood vessels, bladder, skin, muscle etc.). Often, the tissues involved require certain mechanical and structural properties for proper functioning. The term has al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Somatic Cell
A somatic cell (from Ancient Greek σῶμα ''sôma'', meaning "body"), or vegetal cell, is any biological cell forming the body of a multicellular organism other than a gamete, germ cell, gametocyte or undifferentiated stem cell. Such cells compose the body of an organism and divide through the process of binary fission and mitotic division. In contrast, gametes are cells that fuse during sexual reproduction, germ cells are cells that give rise to gametes, and stem cells are cells that can divide through mitosis and differentiate into diverse specialized cell types. For example, in mammals, somatic cells make up all the internal organs, skin, bones, blood and connective tissue, while mammalian germ cells give rise to spermatozoa and ova which fuse during fertilization to produce a cell called a zygote, which divides and differentiates into the cells of an embryo. There are approximately 220 types of somatic cell in the human body. Theoretically, these cells are not germ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene Therapy
Gene therapy is a medical field which focuses on the genetic modification of cells to produce a therapeutic effect or the treatment of disease by repairing or reconstructing defective genetic material. The first attempt at modifying human DNA was performed in 1980, by Martin Cline, but the first successful nuclear gene transfer in humans, approved by the National Institutes of Health, was performed in May 1989. The first therapeutic use of gene transfer as well as the first direct insertion of human DNA into the nuclear genome was performed by French Anderson in a trial starting in September 1990. It is thought to be able to cure many genetic disorders or treat them over time. Between 1989 and December 2018, over 2,900 clinical trials were conducted, with more than half of them in phase I.Gene Therapy Cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Advanced Therapies
A committee or commission is a body of one or more persons subordinate to a deliberative assembly. A committee is not itself considered to be a form of assembly. Usually, the assembly sends matters into a committee as a way to explore them more fully than would be possible if the assembly itself were considering them. Committees may have different functions and their types of work differ depending on the type of the organization and its needs. A member of a legislature may be delegated a committee assignment, which gives them the right to serve on a certain committee. Purpose A deliberative assembly may form a committee (or "commission") consisting of one or more persons to assist with the work of the assembly. For larger organizations, much work is done in committees. Committees can be a way to formally draw together people of relevant expertise from different parts of an organization who otherwise would not have a good way to share information and coordinate actions. They may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Pharmaceutical Regulation
European, or Europeans, or Europeneans, may refer to: In general * ''European'', an adjective referring to something of, from, or related to Europe ** Ethnic groups in Europe ** Demographics of Europe ** European cuisine, the cuisines of Europe and other Western countries * ''European'', an adjective referring to something of, from, or related to the European Union ** Citizenship of the European Union ** Demographics of the European Union In publishing * ''The European'' (1953 magazine), a far-right cultural and political magazine published 1953–1959 * ''The European'' (newspaper), a British weekly newspaper published 1990–1998 * ''The European'' (2009 magazine), a German magazine first published in September 2009 *''The European Magazine'', a magazine published in London 1782–1826 *''The New European'', a British weekly pop-up newspaper first published in July 2016 Other uses * * Europeans (band), a British post-punk group, from Bristol See also * * * Europe (disamb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee On Herbal Medicinal Products
The Committee on Herbal Medicinal Products (HMPC), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on herbal medicines. Role HMPC aims at assisting in the harmonization of procedures and provisions concerning herbal medicinal products within the European Union, and further integrating herbal medicinal products in the European regulatory framework. HMPC provides EU Member States and European institutions with its scientific opinion on questions relating to herbal medicinal products. Other core tasks include the establishment of a draft 'Community list of herbal substances, preparations and combinations thereof for use in traditional herbal medicinal products', as well as the establishment of Community herbal monographs. Composition * 28 members, one nominated by each of the 28 EU Member States The European Union (EU) is a supranational political and economic union of member states that are located primarily in Europe. The u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee On Orphan Medicinal Products
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Veterinary Use
The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged. See also * Committee for Medicinal Products for Human Use The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee A committee or commission is a body of one or more persons subordin ... References External links Committee for Medicinal Products for Veterinary Use (CVMP) Health and the European Union Veterinary organizations Pharmacy Animal health Animal husbandry Medicated feed {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |