|
Etofenamate
Etofenamate is a nonsteroidal anti-inflammatory drug (NSAID) used for the treatment of joint and muscular pain Pain is a distressing feeling often caused by intense or damaging stimuli. The International Association for the Study of Pain defines pain as "an unpleasant sensory and emotional experience associated with, or resembling that associated with, .... It is available for topical application as a cream, a gel or as a spray. Etofenamate is acutely toxic if swallowed; it is also very toxic to aquatic life, with long lasting effects. References Nonsteroidal anti-inflammatory drugs Trifluoromethyl compounds Anthranilates Ethers Primary alcohols {{musculoskeletal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flufenamic Acid
Flufenamic acid (FFA) is a member of the anthranilic acid derivatives (or fenamate) class of nonsteroidal anti-inflammatory drugs (NSAIDs). Like other members of the class, it is a cyclooxygenase (COX) inhibitor, preventing the formation of prostaglandins The prostaglandins (PG) are a group of physiologically active lipid compounds called eicosanoids having diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are deri .... FFA is known to bind to and reduce the activity of prostaglandin F synthase and activate TRPC6. It is not widely used in humans as it has a high rate (30–60%) of gastrointestinal side effects. It is generally not available in the US.NIH LiverTox DatabasMefenamic AcidLast updated June 23, 2015. Page accessed July 3, 2015. Quote: "(fenamates generally not available in the United States, such as tolfenamic acid and flufenamic acid)" It is available in some Asian and Europea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Renal
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder. The kidney participates in the control of the volume of various body fluids, fluid osmolality, acid–base balance, various electrolyte concentrations, and removal of toxins. Filtration occurs in the glomerulus: one-fifth of the blood volume that enters the kidneys is filtered. Examples of substances reabsorbed are solute-free water, sodium, bicarbonate, glucose, and amino acids. Examples of substances secreted are hydrogen, ammonium, potassium and uric acid. The nephron is the structural and functional unit of the kidney. Each adult human kidney contains around 1 million nephrons, while a mouse kidney con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biliary
A bile duct is any of a number of long tube-like structures that carry bile, and is present in most vertebrates. Bile is required for the digestion of food and is secreted by the liver into passages that carry bile toward the hepatic duct. It joins the cystic duct (carrying bile to and from the gallbladder) to form the common bile duct which then opens into the intestine. Structure The top half of the common bile duct is associated with the liver, while the bottom half of the common bile duct is associated with the pancreas, through which it passes on its way to the intestine. It opens into the part of the intestine called the duodenum via the ampulla of Vater. Segments The biliary tree (see below) is the whole network of various sized ducts branching through the liver. The path is as follows: Bile canaliculi → Canals of Hering → interlobular bile ducts → intrahepatic bile ducts → left and right hepatic ducts ''merge to form'' → common hepatic duct ''exits liver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Anti-inflammatory Drug
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease. The term ''non-steroidal'', common from around 1960, distinguishes these drugs from corticosteroids, which during the 1950s had acquired a bad reputation due to overuse and side-effect problems after their initial introduction in 1948. NSAIDs work by inhibiting the activity of cyclooxygenase enzymes (the COX-1 and COX-2 isoenzymes). In cells, these enzymes are involved in the synthesis of key biological mediators, namely prostaglandins, which are involved in inflammation, and thromboxanes, which are involved in blood clotting. There are two general types of NSAIDs available: non-selective, and COX-2 selective. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pain
Pain is a distressing feeling often caused by intense or damaging stimuli. The International Association for the Study of Pain defines pain as "an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage." In medical diagnosis, pain is regarded as a symptom of an underlying condition. Pain motivates the individual to withdraw from damaging situations, to protect a damaged body part while it heals, and to avoid similar experiences in the future. Most pain resolves once the noxious stimulus is removed and the body has healed, but it may persist despite removal of the stimulus and apparent healing of the body. Sometimes pain arises in the absence of any detectable stimulus, damage or disease. Pain is the most common reason for physician consultation in most developed countries. It is a major symptom in many medical conditions, and can interfere with a person's quality of life and general functioning. Simp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PubChem
PubChem is a database of chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information (NCBI), a component of the National Library of Medicine, which is part of the United States National Institutes of Health (NIH). PubChem can be accessed for free through a web user interface. Millions of compound structures and descriptive datasets can be freely downloaded via FTP. PubChem contains multiple substance descriptions and small molecules with fewer than 100 atoms and 1,000 bonds. More than 80 database vendors contribute to the growing PubChem database. History PubChem was released in 2004 as a component of the Molecular Libraries Program (MLP) of the NIH. As of November 2015, PubChem contains more than 150 million depositor-provided substance descriptions, 60 million unique chemical structures, and 225 million biological activity test results (from over 1 million assay experiments performed on mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Anti-inflammatory Drugs
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease. The term ''non-steroidal'', common from around 1960, distinguishes these drugs from corticosteroids, which during the 1950s had acquired a bad reputation due to overuse and side-effect problems after their initial introduction in 1948. NSAIDs work by inhibiting the activity of cyclooxygenase enzymes (the COX-1 and COX-2 isoenzymes). In cells, these enzymes are involved in the synthesis of key biological mediators, namely prostaglandins, which are involved in inflammation, and thromboxanes, which are involved in blood clotting. There are two general types of NSAIDs available: non-selective, and COX-2 selective. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
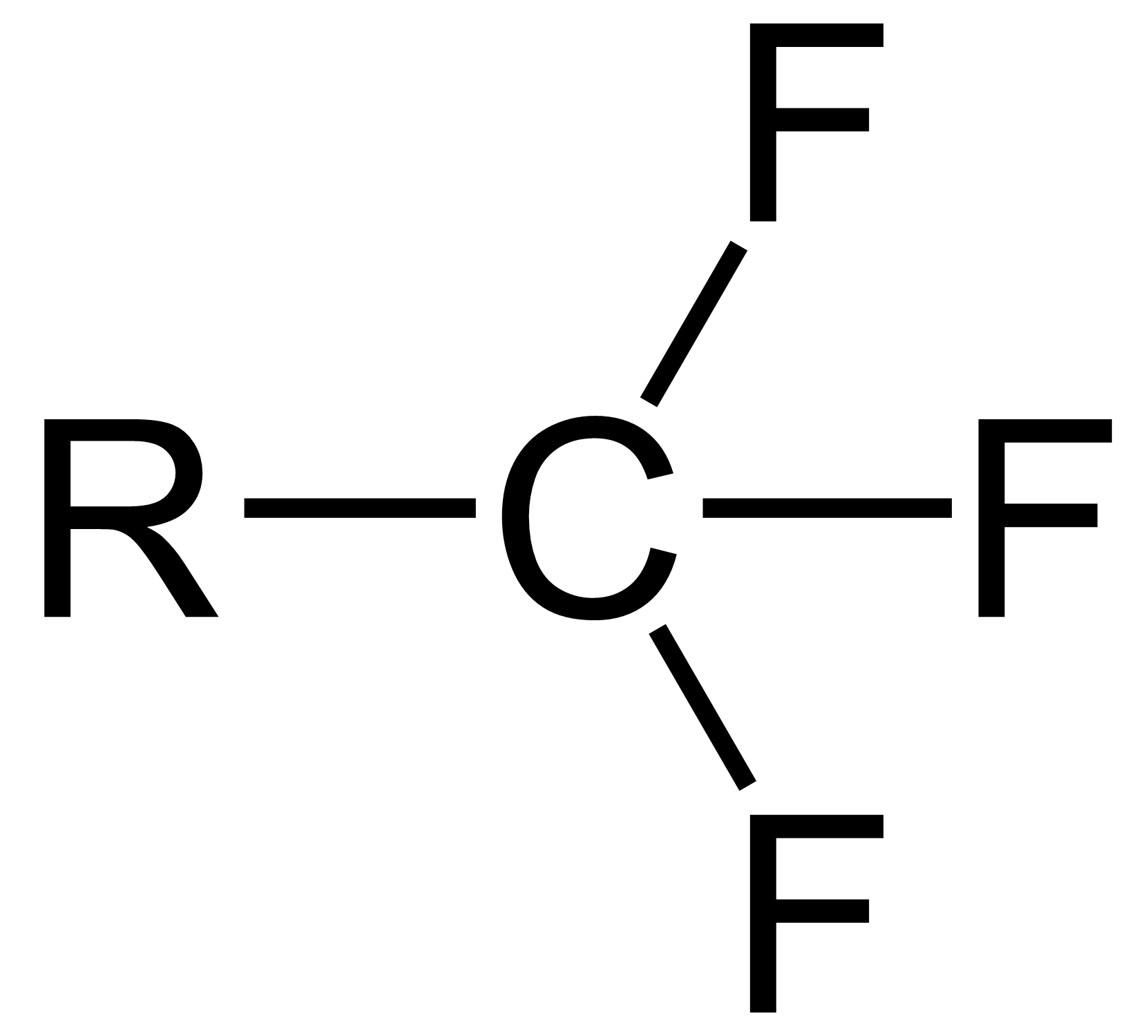
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethers
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |