|
Ergosterol Structure
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a mycosterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the enzymes that synthesize it have become important targets for drug discovery. In human nutrition, ergosterol is a provitamin form of vitamin D2; exposure to ultraviolet (UV) light causes a chemical reaction that produces vitamin D2. Role in fungi Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in fungi, and named after ergot, the common name of members of the fungal genus ''Claviceps'' from which ergosterol was first isolated. Ergosterol is a component of yeast and other fungal cell membranes, serving many of the same functions that cholesterol serves in animal cells. Its specificity in higher fungi is thought to be related to the climatic instabilities (highly varying humidity and moisture conditions) encountered by these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol
A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on C3 position by a hydroxyl group. It is therefore an alcohol (chemistry), alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most Eukaryote, eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to the structure of the cell membrane, and functions as a precursor to fat-soluble vitamins and steroid hormones. While technically alcohols, sterols are classified by biochemists as lipids (f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
African Trypanosomiasis
African trypanosomiasis is an insect-borne parasitic infection of humans and other animals. Human African trypanosomiasis (HAT), also known as African sleeping sickness or simply sleeping sickness, is caused by the species ''Trypanosoma brucei''. Humans are infected by two types, ''Trypanosoma brucei gambiense'' (TbG) and ''Trypanosoma brucei rhodesiense'' (TbR). TbG causes over 92% of reported cases. Both are usually transmitted by the bite of an infected tsetse fly and are most common in rural areas. Initially, the first stage of the disease is characterized by fevers, headaches, itchiness, and joint pains, beginning one to three weeks after the bite. Weeks to months later, the second stage begins with confusion, poor coordination, numbness, and trouble sleeping. Diagnosis involves detecting the parasite in a blood smear or lymph node fluid. A lumbar puncture is often needed to tell the difference between first- and second-stage disease. Prevention of severe disease in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichomonas
''Trichomonas'' is a genus of anaerobic excavate parasites of vertebrates. It was first discovered by Alfred François Donné in 1836 when he found these parasites in the vagina of a patient suffering from vaginitis, an inflammation of the vagina. Donné named the genus from its morphological characteristics. The prefix tricho- originates from the Ancient Greek word (thrix) meaning hair, describing ''Trichomonas''’s flagella. The suffix -monas ( – single unit), describes its similarity to unicellular organisms from the genus ''Monas''. Habitat and ecology ''Trichomonas'' is typically found in anaerobic environments. It is a known parasite of many different animals including humans, birds, dogs, and cats. In humans, it can be found in the urogenital tract and in the oral cavity. It is estimated that 276 million new cases of urogenital infections occur each year. Depending on the ''Trichomonas'' species, it can either be transmitted through direct sexual contact or through ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Demethylation
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen atoms. The counterpart of demethylation is methylation. In biochemistry : Demethylation is relevant to epigenetics. Demethylation of DNA is catalyst, catalyzed by demethylases. These enzymes oxidize N-methyl groups, which occur in histones, in lysine derivatives, and in some forms of DNA. :R2N-CH3 + O → R2N-H + CH2O One family of such oxidative enzymes is the cytochrome P450. Alpha-ketoglutarate-dependent hydroxylases are also active for demethylation of DNA, operating by a similar stoichiometry. These reactions, which proceed via hydroxylation, exploit the slightly weakened Carbon–hydrogen bond, C-H bonds of methylamines and methyl ethers. Demethylation of some sterols are steps in the biosynthesis of testosterone and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitor
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its Enzyme activity, activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which Substrate (biochemistry), substrate molecules are converted into Product (chemistry), products. An enzyme Enzyme catalysis, facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the Rate-determining step, most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind Reversible reaction, reversibly or irreversibly. Irreversible inhibitors form a Covalent bond, chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanosterol 14 Alpha-demethylase
Lanosterol 14α-demethylase (CYP51A1) is the animal version of a cytochrome P450 enzyme that is involved in the conversion of lanosterol to 4,4-dimethylcholesta-8(9),14,24-trien-3β-ol. The cytochrome P450 isoenzymes are a conserved group of proteins that serve as key players in the metabolism of organic substances and the biosynthesis of important steroids, lipids, and vitamins in eukaryotes. As a member of this family, lanosterol 14α-demethylase is responsible for an essential step in the biosynthesis of sterols. In particular, this protein catalyzes the removal of the C-14α-methyl group from lanosterol. This demethylation step is regarded as the initial checkpoint in the transformation of lanosterol to other sterols that are widely used within the cell. Evolution The structural and functional properties of the cytochrome P450 superfamily have been subject to extensive diversification over the course of evolution. Recent estimates indicate that there are currently 10 clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, having a role in maintaining health of the lens. Lanosterol is the precursor to cholesterol. Biosynthesis The biosynthesis of lanosterol has been intensively investigated. Elaboration of lanosterol under enzyme catalysis leads to other steroids. 14-Demethylation of lanosterol by CYP51 eventually yields cholesterol. Research as an eye drop supplement As a molecule naturally enriched in the eye lens, lanosterol is a component involved in maintenance of lens clarity. Its proposed mechanism of action is to inhibit the aggregation of crystallin proteins, which contribute to the clouding of vision by forming cataracts. Lanosterol is under research for its potential as a therapeutic additive in eye drops to inhibit the aggregation of crysta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myclobutanil
Myclobutanil is a triazole chemical used as a fungicide. It is a steroid demethylation ( CYP51) inhibitor, specifically inhibiting ergosterol biosynthesis. Ergosterol is a critical component of fungal cell membranes. Stereoisomerism Safety The Safety Data Sheet indicates the following hazards: *Suspected of damaging fertility or the unborn child. *Toxic to aquatic life with long lasting effects. The first hazard has caused this chemical to be placed on the 1986 California Proposition 65 toxics list. When heated, myclobutanil decomposes to produce corrosive and/or toxic fumes, including carbon monoxide, carbon dioxide, hydrogen chloride, hydrogen cyanide, and nitrogen oxides. Banned for cannabis cultivation Myclobutanil is banned in Canada, Colorado, Washington, Oregon, and Oklahoma for the production of medical and recreational cannabis. In 2014, a Canadian news investigation by ''The Globe and Mail'' reported the discovery of myclobutanil in medical cannabis produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
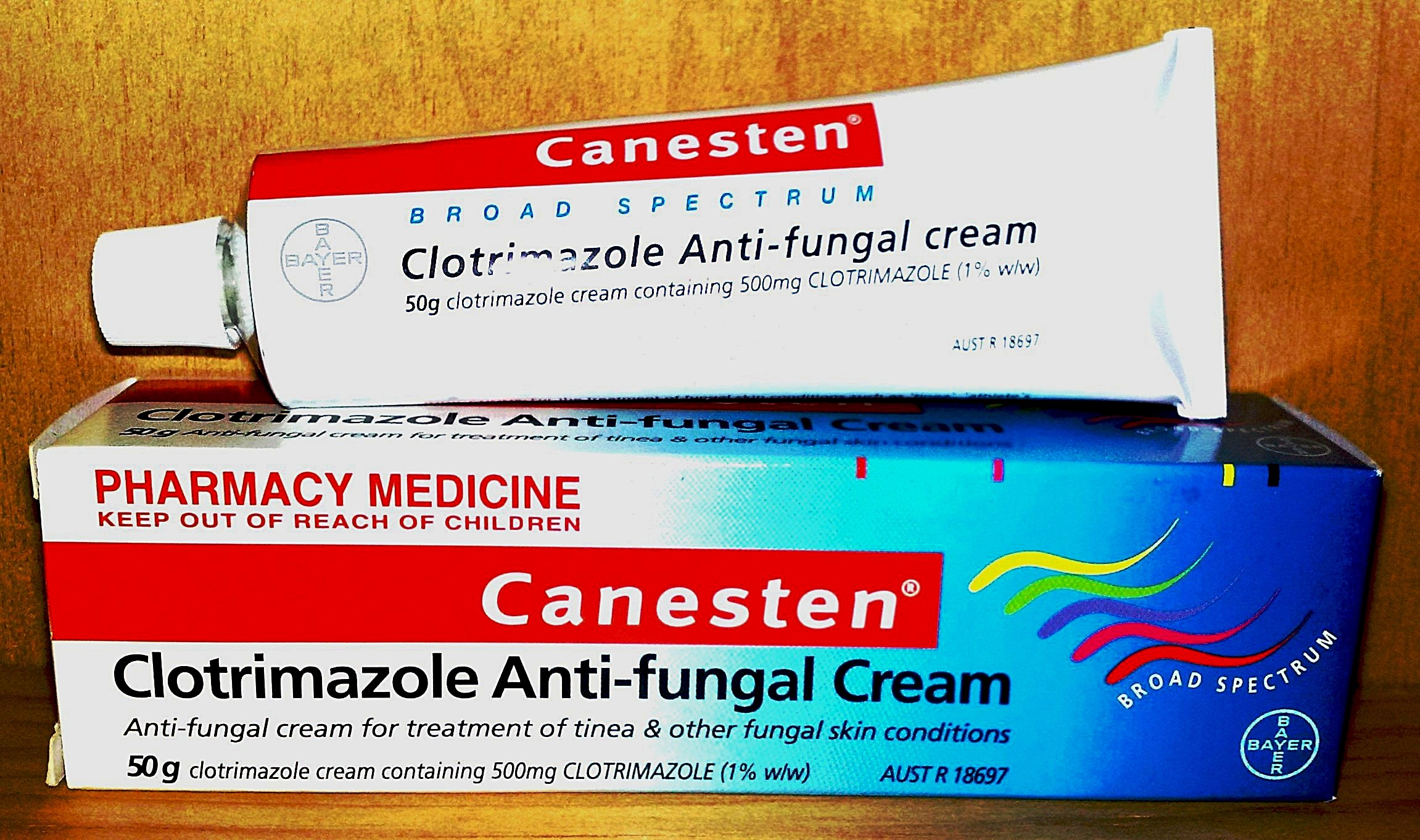
Clotrimazole
Clotrimazole, sold under the brand name Lotrimin, among others, is an antifungal medication. It is used to treat vaginal yeast infections, oral thrush, diaper rash, tinea versicolor, and types of ringworm including athlete's foot and jock itch. It can be taken by mouth or applied as a cream to the skin or in the vagina. Common side effects when taken by mouth include nausea and itchiness. When applied to the skin, common side effects include redness and a burning sensation. In pregnancy, use on the skin or in the vagina is believed to be safe. There is no evidence of harm when used by mouth during pregnancy but this has been less well studied. When used by mouth, greater care should be taken in those with liver problems. It is in the azole class of medications and works by disrupting the fungal cell membrane. Clotrimazole was discovered in 1969. It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. It is available as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Itraconazole
Itraconazole, sometimes abbreviated ITZ, is an antifungal medication used to treat a number of fungal infections. This includes aspergillosis, blastomycosis, coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis. It may be given by mouth or intravenously. Common side effects include nausea, diarrhea, abdominal pain, rash, and headache. Severe side effects may include liver problems, heart failure, Stevens–Johnson syndrome and allergic reactions including anaphylaxis. It is unclear if use during pregnancy or breastfeeding is safe. It is in the triazole family of medications. It stops fungal growth by affecting the cell membrane or affecting their metabolism. Itraconazole was patented in 1978 and approved for medical use in the United States in 1992. It is on the World Health Organization's List of Essential Medicines. Recent research works suggest itraconazole (ITZ) could also be used in the treatment of cancer by inhibiting the hedgehog pathway in a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Miconazole
Miconazole, sold under the brand name Monistat among others, is an antifungal medication used to treat ring worm, pityriasis versicolor, and yeast infections of the skin or vagina. It is used for ring worm of the body, groin (jock itch), and feet (athlete's foot). It is applied to the skin or vagina as a cream or ointment. Common side effects include itchiness or irritation of the area in which it was applied. Use in pregnancy is believed to be safe for the baby. Miconazole is in the imidazole family of medications. It works by decreasing the ability of fungi to make ergosterol, an important part of their cell membrane. Miconazole was patented in 1968 and approved for medical use in 1971. It is on the World Health Organization's List of Essential Medicines. It is available as a generic medication. Medical uses Miconazole is used externally for the treatment of ringworm, jock itch, and athlete's foot. Internal application is used for oral candidiasis or vaginal thrus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluconazole
Fluconazole is an antifungal medication used for a number of fungal infections. These include candidiasis, blastomycosis, coccidioidomycosis, cryptococcosis, histoplasmosis, dermatophytosis, and tinea versicolor. It is also used to prevent candidiasis in those who are at high risk such as following organ transplantation, low birth weight babies, and those with low blood neutrophil counts. It is given either by mouth or by injection into a vein. Common side effects include vomiting, diarrhea, rash, and increased liver enzymes. Serious side effects may include liver problems, QT prolongation, and seizures. During pregnancy it may increase the risk of miscarriage while large doses may cause birth defects. Fluconazole is in the azole antifungal family of medication. It is believed to work by affecting the fungal cellular membrane. Fluconazole was patented in 1981 and came into commercial use in 1988. It is on the World Health Organization's List of Essential M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |