|
Endergonic Reaction
In chemical thermodynamics, an endergonic reaction (; also called a heat absorbing nonspontaneous reaction or an unfavorable reaction) is a chemical reaction in which the standard change in free energy is positive, and an additional driving force is needed to perform this reaction. In layman's terms, the total amount of useful energy is negative (it takes more energy to start the reaction than what is received out of it) so the total energy is a net negative result, as opposed to a net positive result in an exergonic reaction. Another way to phrase this is that useful energy must be absorbed from the surroundings into the workable system for the reaction to happen. Under constant temperature and constant pressure conditions, this means that the change in the standard Gibbs free energy would be positive, :\Delta G^\circ > 0 for the reaction at standard state (i.e. at standard pressure (1 bar), and standard concentrations (1 molar) of all the reagents). In metabolism, an e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion. Thermodynamic temperature is typically expressed using the Kelvin scale, where the unit of measurement is the ''kelvin'' (unit symbol: K). The Kelvin scale uses the same degree interval as the Celsius scale but is offset so that 0 K corresponds to absolute zero. For comparison, a temperature of 295 K corresponds to 21.85 °C and 71.33 °F. Another absolute scale of temperature is the Rankine scale, which is based on the Fahrenheit degree interval. Historically, thermodynamic temperature was defined by Lord Kelvin in terms of a macroscopic relation between Work (thermodynamics), thermodynamic work and Heat, heat transfer as defined in thermodynamics, but the kelvin was redefined by international agreement in 2019 in terms of phenomena that are now understood as man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin (unit)
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By definition, the Celsius scale (symbol °C) and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15. The 19th century British scientist Lord Kelvin first developed and proposed the scale. It was often called the "absolute Celsius" scale in the early 20th century. The kelvin was formally added to the International System of Units in 1954, defining 273.16 K to be the triple point of water. The Celsius, Fahrenheit, and Rankine scales were redefined in terms of the Kelvin scale using this definition. The 2019 revision of the SI now defines the kelvin in terms of energy by setting the Bol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs–Helmholtz Equation
The Gibbs–Helmholtz equation is a thermodynamic equation used to calculate changes in the Gibbs free energy of a system as a function of temperature. It was originally presented in an 1882 paper entitled " Die Thermodynamik chemischer Vorgänge" by Hermann von Helmholtz. It describes how the Gibbs free energy, which was presented originally by Josiah Willard Gibbs, varies with temperature. It was derived by Helmholtz first, and Gibbs derived it only 6 years later. The attribution to Gibbs goes back to Wilhelm Ostwald, who first translated Gibbs' monograph into German and promoted it in Europe. The equation is:Physical chemistry, P. W. Atkins, Oxford University Press, 1978, where ''H'' is the enthalpy, ''T'' the absolute temperature and ''G'' the Gibbs free energy of the system, all at constant pressure ''p''. The equation states that the change in the ''G/T'' ratio at constant pressure as a result of an infinitesimally small change in temperature is a factor ''H/T''2. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endothermic Reaction
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, H.P., Butler, L. J. (2015). '' Principle of Modern Chemistry'', Brooks Cole. p. 617. In an endothermic process, the heat that a system absorbs is thermal energy transfer into the system. Thus, an endothermic reaction generally leads to an increase in the temperature of the system and a decrease in that of the surroundings. The term was coined by 19th-century French chemist Marcellin Berthelot. The term ''endothermic'' comes from the Greek ἔνδον (''endon'') meaning 'within' and θερμ- (''therm'') meaning 'hot' or 'warm'. An endothermic process may be a chemical process, such as dissolving ammonium nitrate () in water (), or a physical process, such as the melting of ice cubes. The opposite of an endothermic process is an exoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change and information systems including the transmission of information in telecommunication. Entropy is central to the second law of thermodynamics, which states that the entropy of an isolated system left to spontaneous evolution cannot decrease with time. As a result, isolated systems evolve toward thermodynamic equilibrium, where the entropy is highest. A consequence of the second law of thermodynamics is that certain processes are irreversible. The thermodynami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Free Energy
In thermodynamics, the thermodynamic free energy is one of the state functions of a thermodynamic system. The change in the free energy is the maximum amount of work that the system can perform in a process at constant temperature, and its sign indicates whether the process is thermodynamically favorable or forbidden. Since free energy usually contains potential energy, it is not absolute but depends on the choice of a zero point. Therefore, only relative free energy values, or changes in free energy, are physically meaningful. The free energy is the portion of any first-law energy that is available to perform thermodynamic work at constant temperature, ''i.e.'', work mediated by thermal energy. Free energy is subject to irreversible loss in the course of such work. Since first-law energy is always conserved, it is evident that free energy is an expendable, second-law kind of energy. Several free energy functions may be formulated based on system criteria. Free energy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spontaneously from hotter to colder regions of matter (or 'downhill' in terms of the temperature gradient). Another statement is: "Not all heat can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. It predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. For example, the first law allows the process of a cup falling off a table and breaking on the floor, as well as allowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
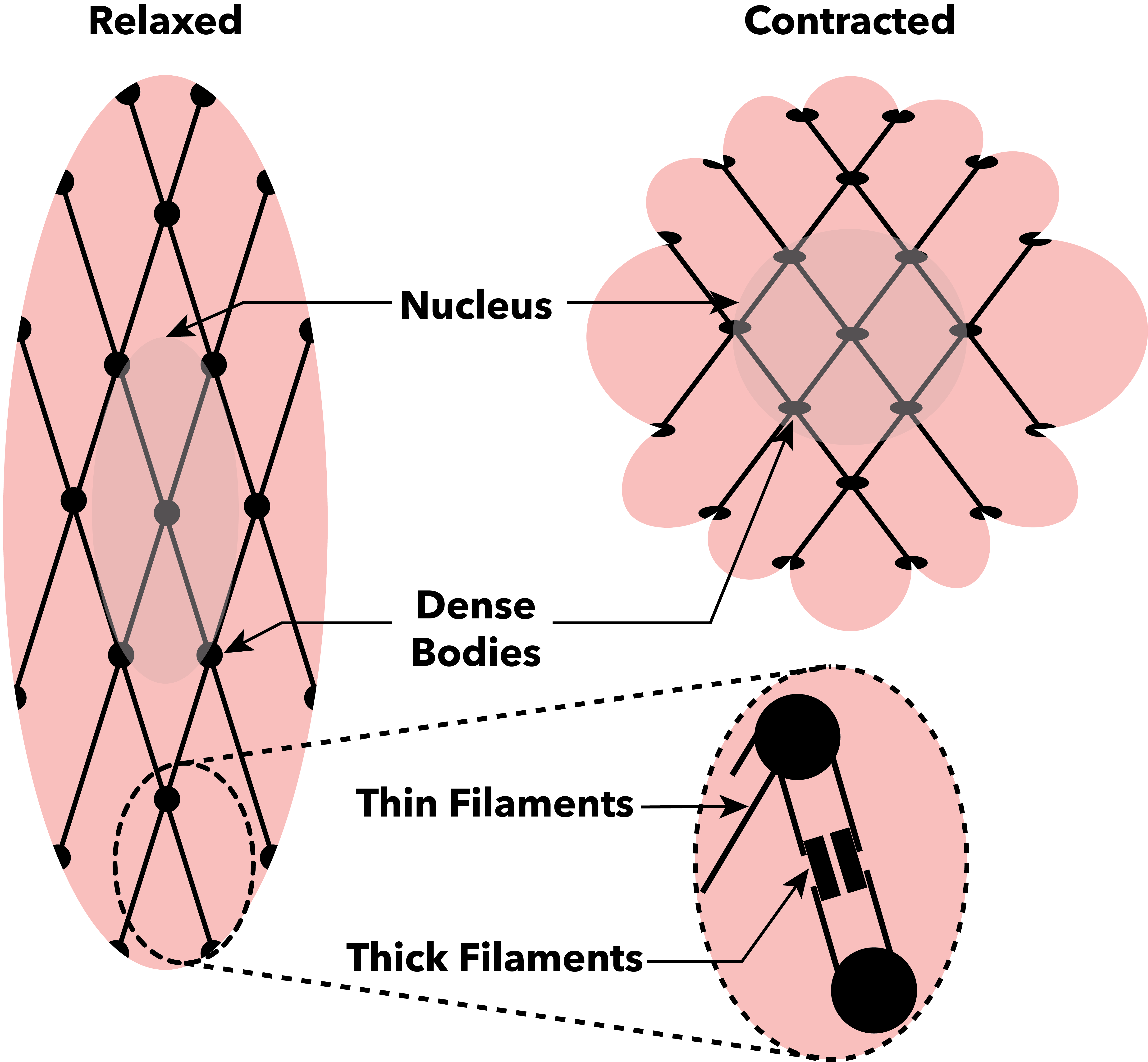
Muscle Contraction
Muscle contraction is the activation of Tension (physics), tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state. For the contractions to happen, the muscle cells must rely on the change in action of two types of Myofilament, filaments: thin and thick filaments. The major constituent of thin filaments is a chain formed by helical coiling of two strands of actin, and thick filaments dominantly consist of chains of the Motor protein, motor-protein myosin. Together, these two filaments form myofibrils - the basic functional organelles in the skeletal muscle system. In vertebrates, Muscle cell#Muscle contraction in striated muscle, skele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
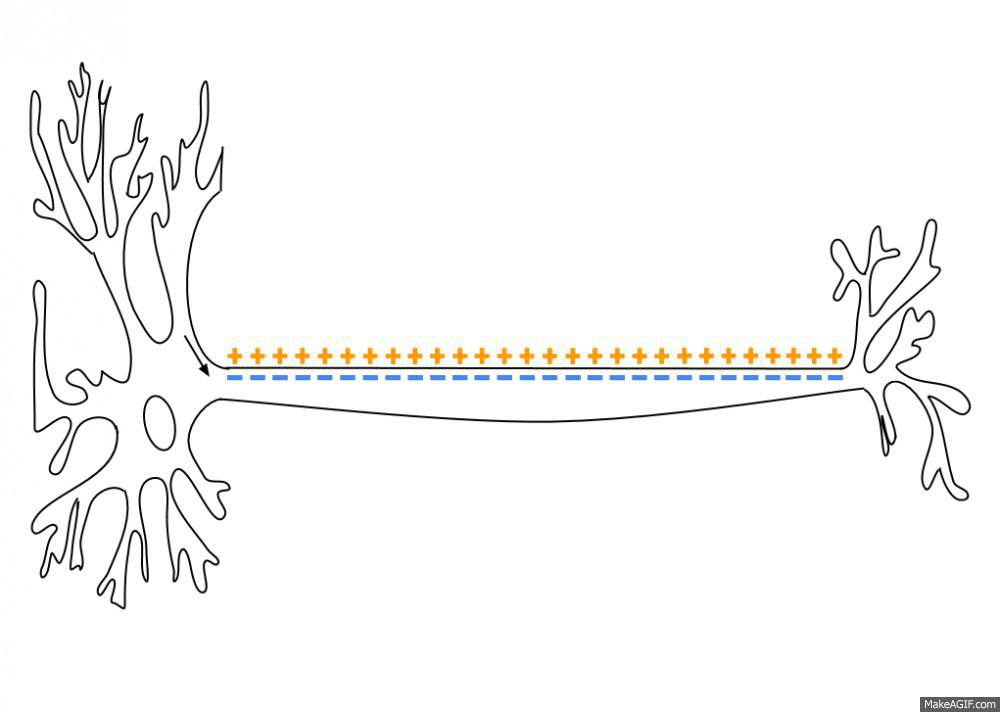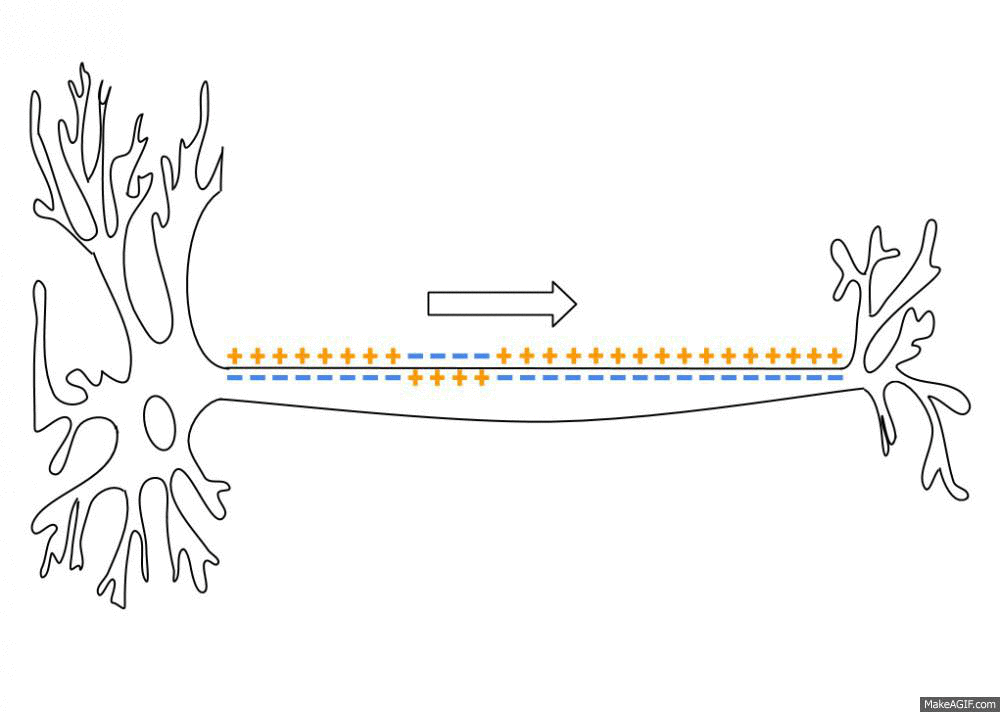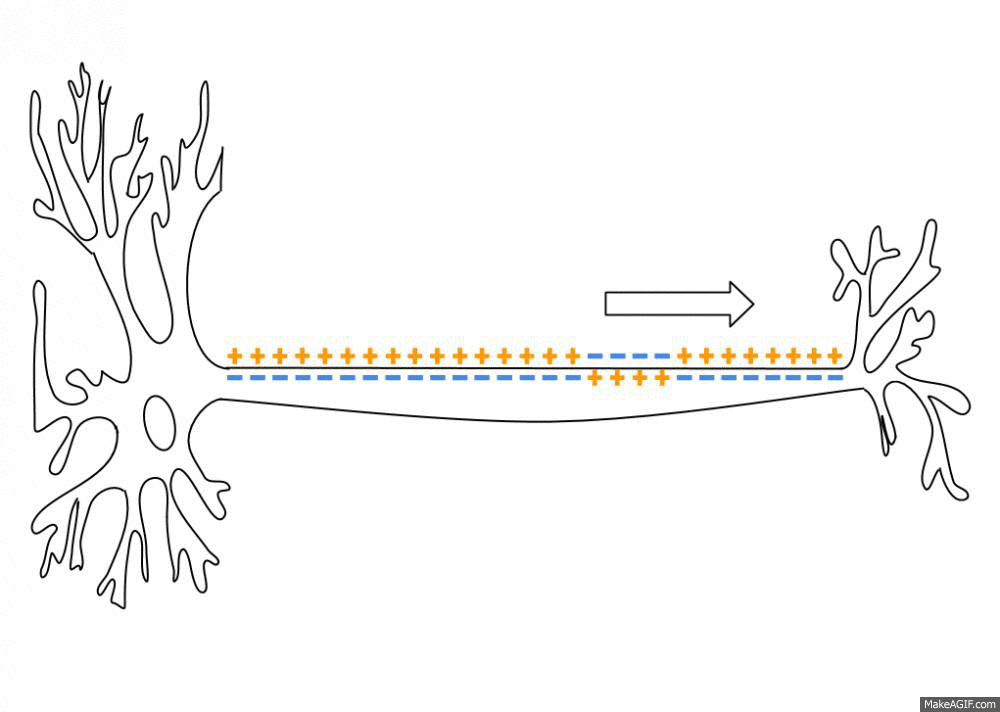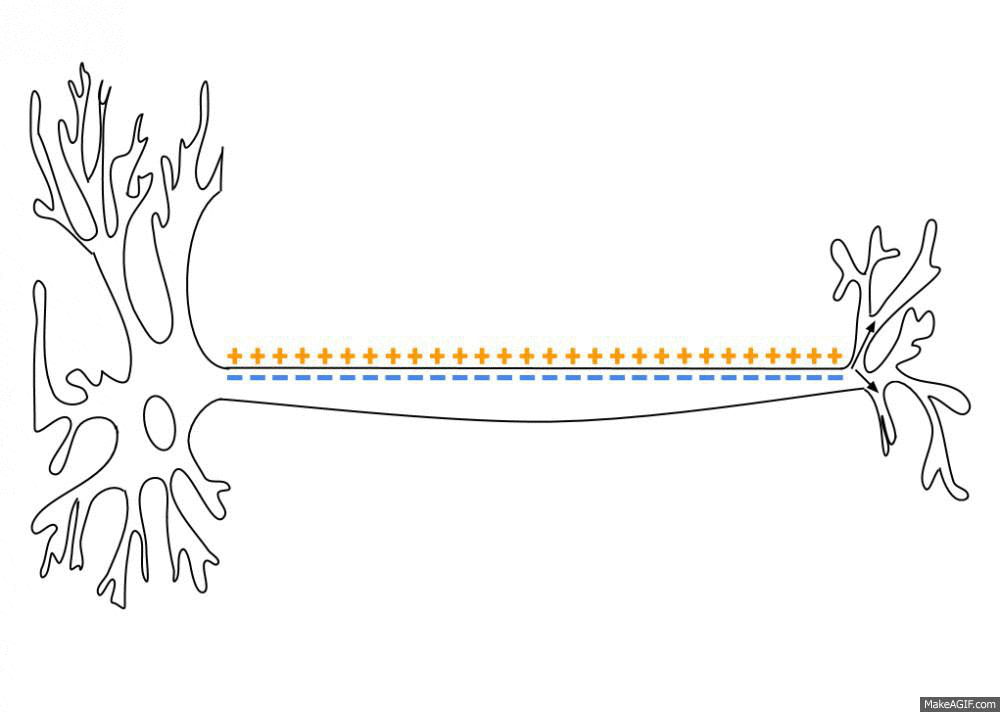
Action Potential
An action potential (also known as a nerve impulse or "spike" when in a neuron) is a series of quick changes in voltage across a cell membrane. An action potential occurs when the membrane potential of a specific Cell (biology), cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of Membrane potential#Cell excitability, excitable cells, which include animal cells like neurons and myocyte, muscle cells, as well as some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells. In neurons, action potentials play a central role in cell–cell interaction, cell–cell communication by providing for—or with regard to saltatory conduction, assisting—the propagation of signals along the neuron's axon toward axon terminal, synaptic boutons situated at the ends of an axon; these signals can then connect wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Synthesis
Protein biosynthesis, or protein synthesis, is a core biological process, occurring inside cells, balancing the loss of cellular proteins (via degradation or export) through the production of new proteins. Proteins perform a number of critical functions as enzymes, structural proteins or hormones. Protein synthesis is a very similar process for both prokaryotes and eukaryotes but there are some distinct differences. Protein synthesis can be divided broadly into two phases: transcription and translation. During transcription, a section of DNA encoding a protein, known as a gene, is converted into a molecule called messenger RNA (mRNA). This conversion is carried out by enzymes, known as RNA polymerases, in the nucleus of the cell. In eukaryotes, this mRNA is initially produced in a premature form (pre-mRNA) which undergoes post-transcriptional modifications to produce mature mRNA. The mature mRNA is exported from the cell nucleus via nuclear pores to the cytoplasm of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |