|
Emulsion
An emulsion is a mixture of two or more liquids that are normally Miscibility, immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms ''colloid'' and ''emulsion'' are sometimes used interchangeably, ''emulsion'' should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase (matter), phase) is dispersion (chemistry), dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working. Two liquids can form different types of emulsions. As an example, oil and water can form, first, an oil-in-water emulsion, in which the oil is the dispersed phase, and water is the continuous phase. Second, they can form a water-in-oil emulsion, in which water is the dispersed phase and oil is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emulsions
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms ''colloid'' and ''emulsion'' are sometimes used interchangeably, ''emulsion'' should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working. Two liquids can form different types of emulsions. As an example, oil and water can form, first, an oil-in-water emulsion, in which the oil is the dispersed phase, and water is the continuous phase. Second, they can form a water-in-oil emulsion, in which water is the dispersed phase and oil is the continuous phase. Multiple emulsions are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
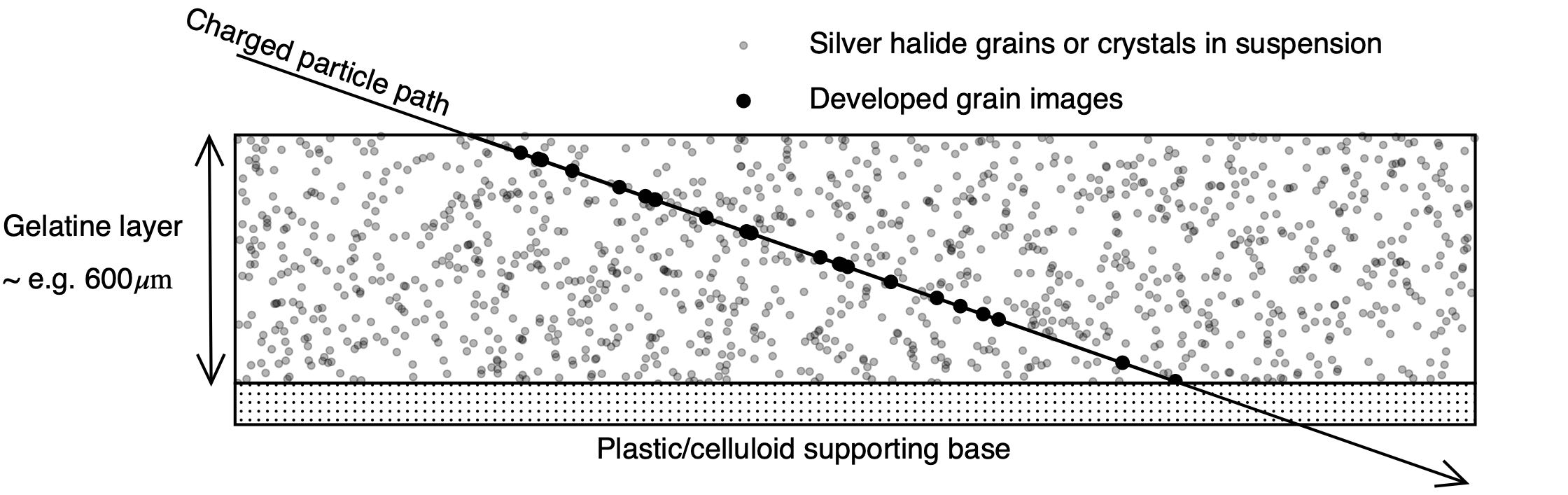
Nuclear Emulsion
A nuclear emulsion plate is a type of particle detector first used in nuclear and particle physics experiments in the early decades of the 20th century. https://cds.cern.ch/record/1728791/files/vol6-issue5-p083-e.pdf''The Study of Elementary Particles by the Photographic Method'', C.F.Powell, P.H.Fowler, D.H.Perkins: Pergamon Press, New York, 1959.Walter H. Barkas, ''Nuclear Research Emulsions I. Techniques and Theory'', in ''Pure and Applied Physics: A Series of Monographs and Textbooks, Vol. 15'', Academic Press, New York and London, 1963. http://becquerel.jinr.ru/text/books/Barkas_NUCL_RES_EMULSIONS.pdf It is a modified form of photographic plate that can be used to record and investigate fast charged particles like alpha-particles, nucleons, leptons or mesons. After exposing and developing the emulsion, single particle tracks can be observed and measured using a microscope. Description The nuclear emulsion plate is a modified form of photographic plate, coated with a thick ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Film
Photographic film is a strip or sheet of transparent film base coated on one side with a gelatin photographic emulsion, emulsion containing microscopically small light-sensitive silver halide crystals. The sizes and other characteristics of the crystals determine the sensitivity, contrast, and image resolution, resolution of the film. Film is typically segmented in ''frames'', that give rise to separate photographs. The emulsion will gradually darken if left exposed to light, but the process is too slow and incomplete to be of any practical use. Instead, a very short exposure (photography), exposure to the image formed by a camera lens is used to produce only a very slight chemical change, proportional to the amount of light absorbed by each crystal. This creates an invisible latent image in the emulsion, which can be chemically photographic processing, developed into a visible photograph. In addition to visible light, all films are sensitive to ultraviolet light, X-rays, gamma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Emulsion
Photographic emulsion is a light-sensitive colloid used in film-based photography. Most commonly, in silver-gelatin photography, it consists of silver halide crystals dispersed in gelatin. The emulsion is usually coated onto a substrate of glass, films (of cellulose nitrate, cellulose acetate or polyester), paper, or fabric. The substrate is often flexible and known as a film base. Photographic emulsion is not a true emulsion, but a suspension of solid particles (silver halide) in a fluid (gelatin in solution). However, the word ''emulsion'' is customarily used in a photographic context. Gelatin or gum arabic layers sensitized with dichromate used in the dichromated colloid processes carbon and gum bichromate are sometimes called ''emulsions''. Some processes do not have emulsions, such as platinum, cyanotype, salted paper, or kallitype. Components Photographic emulsion is a fine suspension of insoluble light-sensitive crystals in a colloid sol, usually consisting of g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dispersion (chemistry)
A dispersion is a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter. Dispersions are classified in a number of different ways, including how large the particles are in relation to the particles of the continuous phase, whether or not precipitation occurs, and the presence of Brownian motion. In general, dispersions of particles sufficiently large for sedimentation are called suspensions, while those of smaller particles are called colloids and solutions. Structure and properties It is widely assumed that dispersions do not display any structure; i.e., the particles (or in case of emulsions: droplets) dispersed in the liquid or solid matrix (the "dispersion medium") are assumed to be statistically distributed. Therefore, for dispersions, usually percolation theory is assumed to appropriately describe their properties. However, percolation theory ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word '' suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study began in 1845 by Franc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomolecular Condensate
In biochemistry, biomolecular condensates are a class of membrane-less organelles and organelle subdomains, which carry out specialized functions within the cell. Unlike many organelles, biomolecular condensate composition is not controlled by a bounding membrane. Instead, condensates can form and maintain organization through a range of different processes, the most well-known of which is phase separation of proteins, RNA, and other biopolymers into either colloidal emulsions, gels, liquid crystals, solid crystals, or aggregates within cells. History Micellar theory The micellar theory of Carl Nägeli was developed from his detailed study of starch granules in 1858. Amorphous substances such as starch and cellulose were proposed to consist of building blocks, packed in a loosely crystalline array to form what he later termed "micelles". Water could penetrate between the micelles, and new micelles could form in the interstices between old micelles. The swelling of st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which can be separated by physical method. It is an impure substance made up of 2 or more elements or compounds mechanically mixed together in any proportion. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions or colloids. Mixtures are one product of mechanically blending or mixing chemical substances such as elements and compounds, without chemical bonding or other chemical change, so that each ingredient substance retains its own chemical properties and makeup. Despite the fact that there are no chemical changes to its constituents, the physical properties of a mixture, such as its melting point, may differ from those of the components. Some mixtures can be separated into their components by using physical (mechanical or thermal) means. Azeotropes are one kind of mixture that usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinaigrette
Vinaigrette ( , ) is made by mixing an edible oil with a mild acid such as vinegar (acetic acid) or lemon juice ( citric acid). The mixture can be enhanced with salt, herbs and/or spices. It is used most commonly as a salad dressing, but can also be used as a marinade. Traditionally, a vinaigrette consists of 3 parts oil and 1 part vinegar mixed into a stable emulsion, but the term is also applied to mixtures with different proportions and to unstable emulsions which last only a short time before separating into layered oil and vinegar phases. Name is the diminutive form of the French word ("vinegar"). It was commonly known as " French dressing" in the 19th century. Preparation In general, vinaigrette consists of 3 parts of oil to 1 part of vinegar whisked into an emulsion. Salt and pepper are often added. Herbs and shallots, too, are often added, especially when it is used for cooked vegetables or grains. Sometimes mustard is used as an emulsifier and to add ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cutting Fluid
Cutting fluid is a type of coolant and lubricant designed specifically for metalworking processes, such as machining and stamping. There are various kinds of cutting fluids, which include oils, oil-water emulsions, pastes, gels, aerosols (mists), and air or other gases. Cutting fluids are made from petroleum distillates, animal fats, plant oils, water and air, or other raw ingredients. Depending on context and on which type of cutting fluid is being considered, it may be referred to as cutting fluid, cutting oil, cutting compound, coolant, or lubricant. Most metalworking and machining processes can benefit from the use of cutting fluid, depending on workpiece material. Common exceptions to this are cast iron and brass, which may be machined dry (though this is not true of all brasses, and any machining of brass will likely benefit from the presence of a cutting fluid). The properties that are sought after in a good cutting fluid are the ability to: * Keep the workpiece at a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to that of a solid, and much higher than that of a gas. Therefore, liquid and solid are classified as condensed matter. Meanwhile, since both liquids and gases can flow, they are categorized as fluids. A liquid is composed of atoms or molecules held together by intermolecular bonds of intermediate strength. These forces allow the particles to move around one another while remaining closely packed. In contrast, solids have particles that are tightly bound by strong intermolecular forces, limiting their movement to small vibrations in fixed positions. Gases, on the other hand, consist of widely spaced, freely moving particles with only weak intermolecular forces. As temperature increases, the molecules in a liquid vibrate more intensely, causi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |