|
Elymoclavine
Elymoclavine is an ergot alkaloid (ergoline alkaloid). It can be produced from ''C. fusiformis'' from ''Pennisetum typhoideum''. It is a precursor in the biosynthesis of D-(+)-lysergic acid. Ergot alkaloids are natural products derived from L-tryptophan. They are often toxic for humans and animals. Despite that they are also well known for their pharmacological activities. The compound is described as being non-hallucinogenic in humans, instead producing mainly sedative effects, and as not contributing to the psychoactive or hallucinogenic effects of morning glory seeds. The doses employed were not provided. Biosynthesis The main building blocks for biosynthesis of elymoclavine are tryptophan (Trp) and DMAPP. DMATrp is obtained after electrophilic substitution followed by addition (Step A below). Then an amine is methylated by an N-methyltransfersase (Step B). Next, the allyl alcohol is oxidized to the diene (Step C). After 1,4-elimination, the diene undergoes an epoxidation ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chanoclavine
Chanoclavine, also known as chanoclavin-I, is a tricyclic ergot alkaloid (ergoline) isolate of certain fungi. It is mainly produced by members of the genus ''Claviceps''. Long used in traditional Chinese medicine, it was found in 1987 mouse studies to stimulate dopamine D2 receptors in the brain. It is described as being devoid of ergot-like activity, possessing no outstanding pharmacological activity, and as not contributing to the hallucinogenic effects of morning glory Morning glory (also written as morning-glory) is the common name for over 1,000 species of flowering plants in the family Convolvulaceae, whose taxonomy and systematics remain in flux. These species are distributed across numerous genus, gene ... seeds. See also * Chanoclavine II * Chanoclavine-I dehydrogenase References Partial ergolines {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ergot Alkaloid
Ergoline is a core structure in many alkaloids and their synthetic derivatives. Ergoline alkaloids were first characterized in ergot. Some of these are implicated in the condition of ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives. Others, such as lysergic acid diethylamide, better known as LSD, a Semisynthesis, semi-synthetic derivative, and ergine, a natural derivative found in ''Argyreia nervosa'', ''Ipomoea tricolor'' and related species, are known Psychedelic drug, psychedelic substances. Natural occurrence Ergoline alkaloids are found in fungi such as Claviceps purpurea, Claviceps paspali, and the related Periglandula, which have a permanent, symbiotic bond with numerous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agroclavine
Agroclavine belongs to the group of ergot alkaloids, which also includes ergotamine Ergotamine, sold under the brand name Ergomar among others, is an ergopeptine and part of the ergot family of alkaloids; it is structurally and biochemically closely related to ergoline. It is structurally similar to several neurotransmitter .... Historically, the main use of agroclavine was in the synthesis of ergot-based drugs; agroclavine can be oxidized to elymoclavine, which then undergoes further processing. References {{ergolines Ergot alkaloids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysergic Acid
Lysergic acid, also known as -lysergic acid and (+)-lysergic acid, is a precursor for a wide range of ergoline alkaloids that are produced by the ergot fungus and found in the seeds of '' Argyreia nervosa'' ( Hawaiian baby woodrose), and ''Ipomoea'' species ( morning glories, ololiuhqui, tlitliltzin). Amides of lysergic acid, lysergamides, are widely used as pharmaceuticals and as psychedelic drugs, e.g. lysergic acid diethylamide (LSD). Lysergic acid is listed as a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. The name Lysergic acid comes from the fact that it is a carboxylic acid, and it was first made by hydrolysis of various ergot alkaloids. Synthesis Laboratory Lysergic acid is generally produced by hydrolysis of natural lysergamides, but can also be synthesized in the laboratory by a complex total synthesis, for example by Robert Burns Woodward's team in 1956. An enantioselective total ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hallucinogen
Hallucinogens, also known as psychedelics, entheogens, or historically as psychotomimetics, are a large and diverse class of psychoactive drugs that can produce altered states of consciousness characterized by major alterations in thought, mood, and perception as well as other changes. Hallucinogens are often categorized as either being psychedelics, dissociatives, or deliriants, but not all hallucinogens fall into these three classes. Examples of hallucinogens include psychedelics or serotonin 5-HT2A receptor agonists like LSD, psilocybin, mescaline, and DMT; dissociatives or NMDA receptor antagonists like ketamine, PCP, DXM, and nitrous oxide; deliriants or antimuscarinics like scopolamine and diphenhydramine; cannabinoids or cannabinoid CB1 receptor agonists like THC, nabilone, and JWH-018; κ-opioid receptor agonists like salvinorin A and pentazocine; GABAA receptor agonists like muscimol and gaboxadol; and oneirogens like ibogaine and harmaline, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sedative
A sedative or tranquilliser is a substance that induces sedation by reducing irritability or Psychomotor agitation, excitement. They are central nervous system (CNS) Depressant, depressants and interact with brain activity, causing its deceleration. Various kinds of sedatives can be distinguished, but the majority of them affect the neurotransmitter Gamma-Aminobutyric acid, gamma-aminobutyric acid (GABA). Most sedatives produce relaxing effects by increasing GABA activity. This group is related to hypnotics. The term ''sedative'' describes drugs that serve to calm or Anxiolytic, relieve anxiety, whereas the term ''hypnotic'' describes drugs whose main purpose is to initiate, sustain, or lengthen sleep. Because these two functions frequently overlap, and because drugs in this class generally produce dose-dependent effects (ranging from anxiolysis to loss of consciousness), they are often referred to collectively as ''sedative–hypnotic'' drugs. Terminology There is some overlap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychoactive Drug
A psychoactive drug, psychopharmaceutical, mind-altering drug, consciousness-altering drug, psychoactive substance, or psychotropic substance is a chemical substance that alters psychological functioning by modulating central nervous system activity. Psychoactive and psychotropic drugs both affect the brain, with psychotropics sometimes referring to psychiatric drugs or high-abuse substances, while “drug” can have negative connotations. Designer drug, Novel psychoactive substances are designer drugs made to mimic illegal ones and bypass laws. Psychoactive drug use dates back to prehistory for medicinal and consciousness-altering purposes, with evidence of widespread cultural use. Many animals intentionally consume psychoactive substances, and some traditional legends suggest animals first introduced humans to their use. Psychoactive substances are used across cultures for purposes ranging from medicinal and therapeutic treatment of Mental disorder, mental disorders and pain, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3 (niacin). It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Tryptophan is named after the digestive enzymes trypsin, which were used in its first isolation from casein proteins. It was assigned the one-letter symbol W based on the double ring being visually suggestive to the bulky letter. Function ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DMAPP
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynthesis. It is an isomer of isopentenyl pyrophosphate (IPP) and exists in virtually all life forms. The enzyme isopentenyl pyrophosphate isomerase catalyzes isomerization between DMAPP and IPP. In the mevalonate pathway, DMAPP is synthesised from mevalonic acid. In contrast, DMAPP is synthesised from HMBPP in the MEP pathway. At present, it is believed that there is crossover between the two pathways in organisms that use both pathways to create terpenes and terpenoid The terpenoids, also known as isoprenoids, are a class of naturally occurring organic compound, organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeabl ...s, such as in plants, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
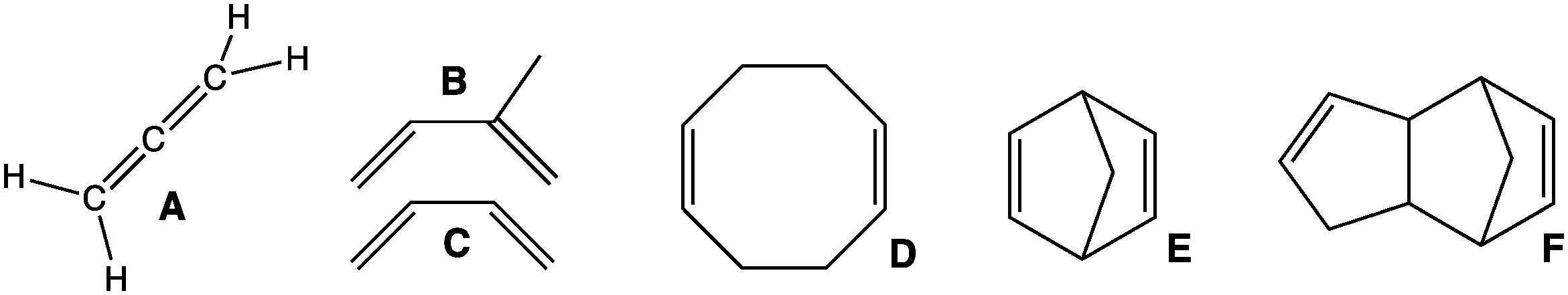
Diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition. Classes Dienes can be divided into three classes, depending on the relative location of the double bonds: #Cumulated dienes have the double bonds sharing a common atom. The result is more specifically called an allene. #Conjugated dienes have conjugated double bonds separated by one single bond. Conjugated dienes are more stable than other dienes because of resonance. #Unconjugated dienes have the double bonds separated by two or more single bonds. They are usually less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxidation
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy''. However, few if any of the epoxy groups in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: : Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Overall, decarboxylation depends upon stability of the carbanion synthon , although the anion may not be a true chemical intermediate. Typically, carboxylic acids decarboxylate slowly, but carboxylic acids with an α el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |