|
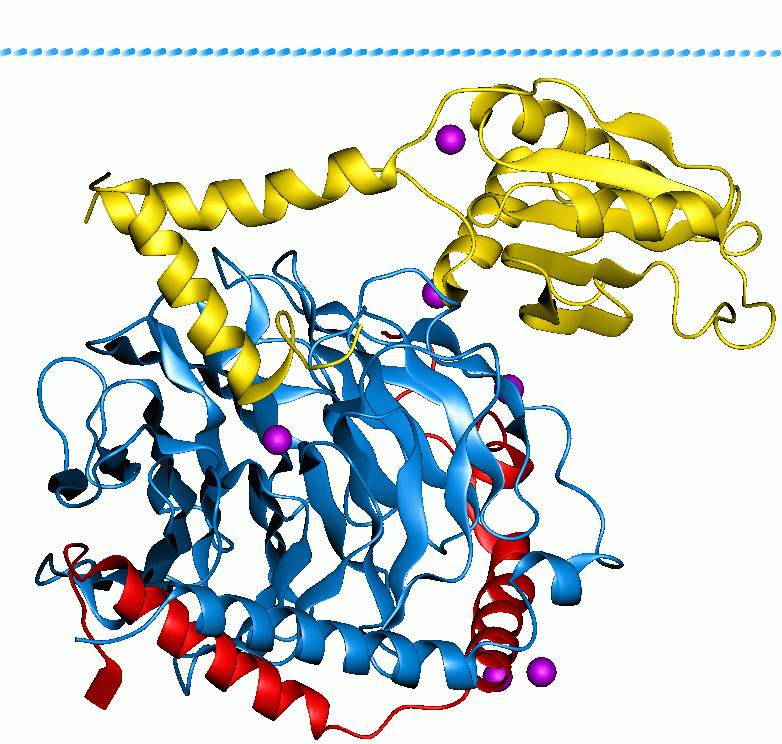
ELMO (protein)
ELMO (Engulfment and Cell Motility) is a family of related proteins (~82 kDa) involved in intracellular signalling networks. These proteins have no intrinsic catalytic activity and instead function as adaptors which can regulate the activity of other proteins through their ability to mediate protein-protein interactions. This family contains members in all animals. In humans there are three paralogous isoforms: *ELMO1 * ELMO2 * ELMO3 The ELMO domain was first characterized in the CED-12 proteins of ''Caenorhabditis elegans'' and ''Drosophila melanogaster'', which is a homolog to the ELMO protein found in mammals. This protein is involved in Rac-GTPase activation, apoptotic cell phagocytosis, cell migration, and cytoskeletal rearrangements. Structure and function of ELMO proteins The ELMO family are evolutionarily conserved orthologs of the ''C. elegans'' protein CED-12. All isoforms contain a series of armadillo repeats, which begin at the N-terminus and extend around ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
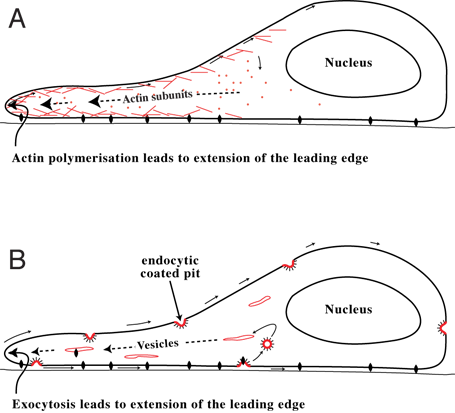
Cell Migration
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryogenesis, embryonic development, wound healing and immune system, immune responses all require the orchestrated movement of cells in particular directions to specific locations. Cells often migrate in response to specific external signals, including chemotaxis, chemical signals and mechanotaxis, mechanical signals. Errors during this process have serious consequences, including intellectual disability, cardiovascular disease, vascular disease, tumor, tumor formation and metastasis. An understanding of the mechanism by which cells migrate may lead to the development of novel therapeutic strategies for controlling, for example, invasive tumour cells. Due to the highly viscous environment (low Reynolds number), cells need to continuously produce forces in order to move. Cells achieve active movement by very different mechanisms. Many less complex prokaryotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Proteins
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of ''alpha'' (Gα), ''beta'' (Gβ) and ''gamma'' (Gγ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rho Family
The Rho family of GTPases is a family of small (~21 kDa) signaling G proteins, and is a subfamily of the Ras superfamily. The members of the Rho GTPase family have been shown to regulate many aspects of intracellular actin dynamics, and are found in all eukaryotic kingdoms, including yeasts and some plants. Three members of the family have been studied in detail: Cdc42, Rac1, and RhoA. All G proteins are "molecular switches", and Rho proteins play a role in organelle development, cytoskeletal dynamics, cell movement, and other common cellular functions. History Identification of the Rho family of GTPases began in the mid-1980s. The first identified Rho member was RhoA, isolated serendipitously in 1985 from a low stringency cDNA screening. Rac1 and Rac2 were identified next, in 1989 followed by Cdc42 in 1990. Eight additional mammalian Rho members were identified from biological screenings until the late 1990s, a turning point in biology where availability of complete genome sequenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanine Nucleotide Exchange Factor
Guanine nucleotide exchange factors (GEFs) are proteins or protein domains that activate monomeric GTPases by stimulating the release of guanosine diphosphate (GDP) to allow binding of guanosine triphosphate (GTP). A variety of unrelated structural domains have been shown to exhibit guanine nucleotide exchange activity. Some GEFs can activate multiple GTPases while others are specific to a single GTPase. Function Guanine nucleotide exchange factors (GEFs) are proteins or protein domains involved in the activation of small GTPases. Small GTPases act as molecular switches in intracellular signaling pathways and have many downstream targets. The most well-known GTPases comprise the Ras superfamily and are involved in essential cell processes such as cell differentiation and proliferation, cytoskeletal organization, vesicle trafficking, and nuclear transport. GTPases are active when bound to GTP and inactive when bound to GDP, allowing their activity to be regulated by GEFs and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dock180
Dedicator of cytokinesis protein 1 (Dock1), also (DOCK180), is a large (~180 kDa) protein encoded in the human by the ''DOCK1'' gene, involved in intracellular signalling networks. It is the mammalian ortholog of the ''C. elegans'' protein CED-5 and belongs to the DOCK family of guanine nucleotide exchange factors (GEFs). Discovery DOCK180 was identified, using a far-western blotting approach, as a binding partner of the adaptor protein Crk that was able to induce morphological changes in 3T3 fibroblasts. Subsequently it was reported that DOCK180 was able to activate the small GTP-binding protein (G protein) Rac1 and this was later shown to happen via its ability to act as a GEF. Structure and function DOCK180 is part of a large class of proteins (GEFs) which contribute to cellular signalling events by activating small G proteins. In their resting state G proteins are bound to Guanosine diphosphate (GDP) and their activation requires the dissociation of GDP and binding of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multidomain enzymes, in which multiple active site, catalytic domains are found in a single polypeptide chain. Protein complexes are a form of protein quaternary structure, quaternary structure. Proteins in a protein complex are linked by non-covalent interactions, non-covalent protein–protein interactions. These complexes are a cornerstone of many (if not most) biological processes. The cell is seen to be composed of modular supramolecular complexes, each of which performs an independent, discrete biological function. Through proximity, the speed and selectivity of binding interactions between Enzyme, enzymatic complex and substrates can be vastly improved, leading to higher cellular efficiency. Many of the techniques used to enter cells and isolate proteins are inherently disruptive to such large complexes, complicating the task of determining ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PH Domain
Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosphatidylinositol lipids within biological membranes (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate), and proteins such as the βγ-subunits of heterotrimeric G proteins, and protein kinase C. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. Lipid binding specificity Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e.g., some bind phosphatidylinositol (4,5)-bisphosphate but not phosphatidylinositol (3,4,5)-trisphosphate or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Armadillo Repeats
An armadillo repeat is a characteristic, repetitive amino acid peptide sequence, sequence of about 42 residue (chemistry)#biochemistry, residues in length that is found in many proteins. Proteins that contain armadillo repeats typically contain several Protein tandem repeats, tandemly repeated copies. Each armadillo repeat is composed of a pair of alpha helices that form a hairpin structure. Multiple copies of the repeat form what is known as an alpha solenoid structure. Examples of proteins that contain armadillo repeats include beta catenin, β-catenin, Sarm1 (SARM1), importin α, α-importin, plakoglobin, adenomatous polyposis coli (APC), and many others. The term armadillo derives from the historical name of the β-catenin gene in the fruitfly ''Drosophila melanogaster, Drosophila'', where the armadillo repeat was first discovered. Although β-catenin was previously believed to be a protein involved in linking cadherin cell adhesion proteins to the cytoskeleton, recent work in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |