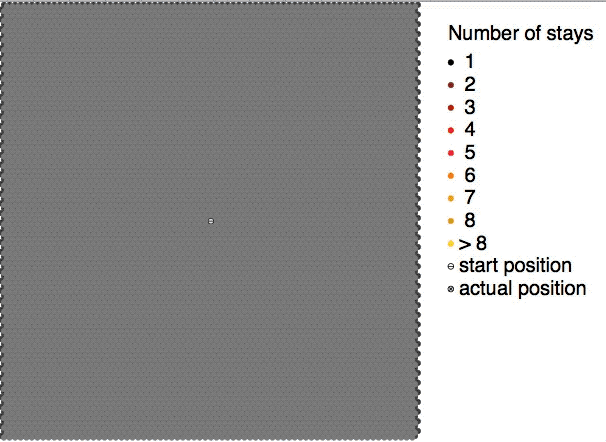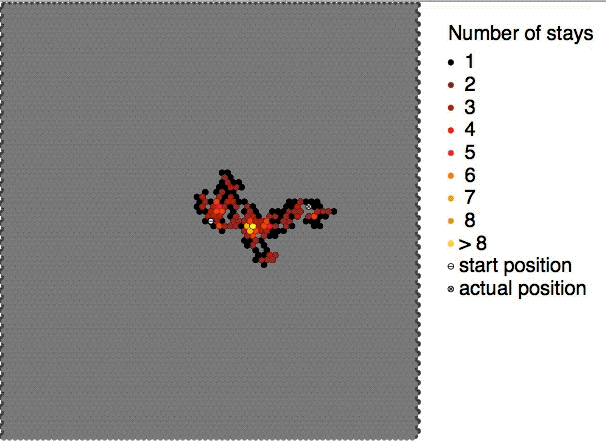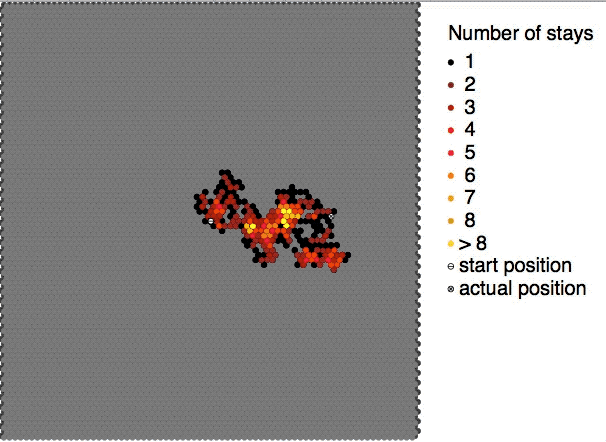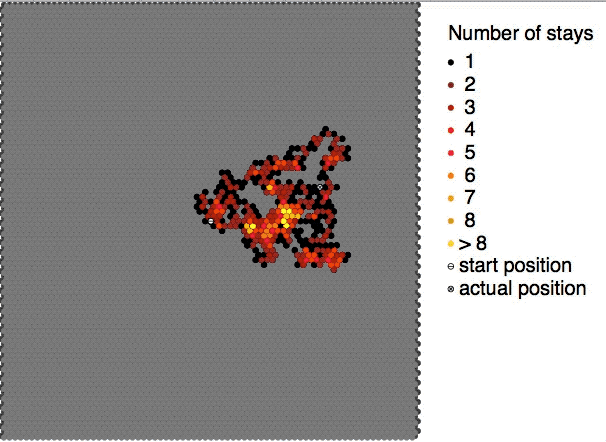
|
Drude Model
The Drude model of electrical conduction was proposed in 1900 by Paul Drude to explain the transport properties of electrons in materials (especially metals). Basically, Ohm's law was well established and stated that the current and voltage driving the current are related to the resistance of the material. The inverse of the resistance is known as the conductance. When we consider a metal of unit length and unit cross sectional area, the conductance is known as the conductivity, which is the inverse of resistivity. The Drude model attempts to explain the resistivity of a conductor in terms of the scattering of electrons (the carriers of electricity) by the relatively immobile ions in the metal that act like obstructions to the flow of electrons. The model, which is an application of kinetic theory, assumes that the microscopic behaviour of electrons in a solid may be treated classically and behaves much like a pinball machine, with a sea of constantly jittering electrons b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Einstein Solid
The Einstein solid is a model of a crystalline solid that contains a large number of independent three-dimensional quantum harmonic oscillators of the same frequency. The independence assumption is relaxed in the Debye model. While the model provides qualitative agreement with experimental data, especially for the high-temperature limit, these oscillations are in fact phonons, or collective modes involving many atoms. Albert Einstein was aware that getting the frequency of the actual oscillations would be difficult, but he nevertheless proposed this theory because it was a particularly clear demonstration that quantum mechanics could solve the specific heat problem in classical mechanics. Historical impact The original theory proposed by Einstein in 1907 has great historical relevance. The heat capacity of solids as predicted by the empirical Dulong–Petit law was required by classical mechanics, the specific heat of solids should be independent of temperature. But experiment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plum Pudding Model
The plum pudding model is an obsolete scientific model of the atom. It was first proposed by J. J. Thomson in 1904 following his discovery of the electron in 1897, and was rendered obsolete by Ernest Rutherford's discovery of the atomic nucleus in 1911. The model tried to account for two properties of atoms then known: that there are electrons, and that atoms have no net electric charge. Logically there had to be an equal amount of positive charge to balance out the negative charge of the electrons. As Thomson had no idea as to the source of this positive charge, he tentatively proposed that it was everywhere in the atom, and that the atom was spherical. This was the mathematically simplest hypothesis to fit the available evidence, or lack thereof. In such a sphere, the negatively charged electrons would distribute themselves in a more or less even manner throughout the volume, simultaneously repelling each other while being attracted to the positive sphere's center. Despite Thoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence formula , which arises from special relativity, has been called "the world's most famous equation". He received the 1921 Nobel Prize in Physics for . Born in the German Empire, Einstein moved to Switzerland in 1895, forsaking his German citizenship (as a subject of the Kingdom of Württemberg) the following year. In 1897, at the age of seventeen, he enrolled in the mathematics and physics teaching diploma program at the Swiss ETH Zurich, federal polytechnic school in Zurich, graduating in 1900. He acquired Swiss citizenship a year later, which he kept for the rest of his life, and afterwards secured a permanent position at the Swiss Patent Office in Bern. In 1905, he submitted a successful PhD dissertation to the University of Zurich. In 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avogadro Number
The Avogadro constant, commonly denoted or , is an SI defining constant with an exact value of when expressed in reciprocal moles. It defines the ratio of the number of constituent particles to the amount of substance in a sample, where the particles in question are any designated elementary entity, such as molecules, atoms, ions, ion pairs. The numerical value of this constant is known as the Avogadro number, commonly denoted . The Avogadro ''number'' is an exact number equal to the number of constituent particles in one mole of any substance (by definition of the mole), historically derived from the experimental determination of the number of atoms in 12 grams of carbon-12 (12C) before the 2019 revision of the SI. Both the constant and the number are named after the Italian physicist and chemist Amedeo Avogadro. The Avogadro constant is used as a proportionality factor in relating the ''amount of substance'' , in a sample of a substance , to the corresponding num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brownian Motion
Brownian motion is the random motion of particles suspended in a medium (a liquid or a gas). The traditional mathematical formulation of Brownian motion is that of the Wiener process, which is often called Brownian motion, even in mathematical sources. This motion pattern typically consists of Randomness, random fluctuations in a particle's position inside a fluid sub-domain, followed by a relocation to another sub-domain. Each relocation is followed by more fluctuations within the new closed volume. This pattern describes a fluid at thermal equilibrium, defined by a given temperature. Within such a fluid, there exists no preferential direction of flow (as in transport phenomena). More specifically, the fluid's overall Linear momentum, linear and Angular momentum, angular momenta remain null over time. The Kinetic energy, kinetic energies of the molecular Brownian motions, together with those of molecular rotations and vibrations, sum up to the caloric component of a fluid's in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wiedemann–Franz Law
In physics, the Wiedemann–Franz law states that the ratio of the electronic contribution of the thermal conductivity (''κ'') to the electrical conductivity (''σ'') of a metal is proportional to the temperature (''T''). : \frac \kappa \sigma = LT Theoretically, the proportionality constant ''L'', known as the Lorenz number, is equal to : L = \frac \kappa = \frac 3 \left(\frac e \right)^2 = 2.44\times 10^\;\mathrm^, where ''k''B is the Boltzmann constant and ''e'' is the elementary charge. This empirical law is named after Gustav Wiedemann and Rudolph Franz, who in 1853 reported that ''κ''/''σ'' has approximately the same value for different metals at the same temperature. The proportionality of ''κ''/''σ'' with temperature was discovered by Ludvig Lorenz in 1872. Derivation Qualitatively, this relationship is based upon the fact that the heat and electrical transport both involve the free electrons in the metal. The mathematical expre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Drude
Paul Karl Ludwig Drude (; ; 12 July 1863 – 5 July 1906) was a German physicist specializing in optics. He was known for the Drude model. Biography Education Born in Braunschweig, Drude began his studies in mathematics at the University of Göttingen, but later changed his major to physics. His dissertation covering the reflection and diffraction of light in crystals was completed in 1887, under Woldemar Voigt. Drude graduated the year Heinrich Hertz began publishing his findings from his experiments on the electromagnetic theories of James Clerk Maxwell. Thus Drude began his professional career at the time Maxwell's theories were being introduced into Germany. Career In 1894, Drude became an extraordinarius professor at the University of Leipzig; in the same year he married Emilie Regelsberger, daughter of a Göttingen lawyer. They had four children. In 1900, he became the editor for ''Annalen der Physik'', the most respected physics journal at that time. From 1901 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiclassical Physics
In physics, semiclassical refers to a theory in which one part of a system is described quantum mechanically, whereas the other is treated classically. For example, external fields will be constant, or when changing will be classically described. In general, it incorporates a development in powers of the Planck constant, resulting in the classical physics of power 0, and the first nontrivial approximation to the power of (−1). In this case, there is a clear link between the quantum-mechanical system and the associated semi-classical and classical approximations, as it is similar in appearance to the transition from physical optics to geometric optics. History Max Planck was the first to introduce the idea of quanta of energy in 1900 while studying black-body radiation. In 1906, he was also the first to write that quantum theory should replicate classical mechanics at some limit, particularly if the Planck constant ''h'' were infinitesimal. With this idea he showed tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sommerfeld
Arnold Johannes Wilhelm Sommerfeld (; 5 December 1868 – 26 April 1951) was a German theoretical physicist who pioneered developments in atomic and quantum physics, and also educated and mentored many students for the new era of theoretical physics. He served as doctoral advisor and postdoc advisor to seven Nobel Prize winners and supervised at least 30 other famous physicists and chemists. Only J. J. Thomson's record of mentorship offers a comparable list of high-achieving students. He introduced the second quantum number, azimuthal quantum number, and the third quantum number, magnetic quantum number. He also introduced the fine-structure constant and pioneered X-ray wave theory. Early life and education Sommerfeld was born in 1868 to a family with deep ancestral roots in Prussia. His mother Cäcilie Matthias (1839–1902) was the daughter of a Potsdam builder. His father Franz Sommerfeld (1820–1906) was a physician from a leading family in Königsberg, where Arnold's g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
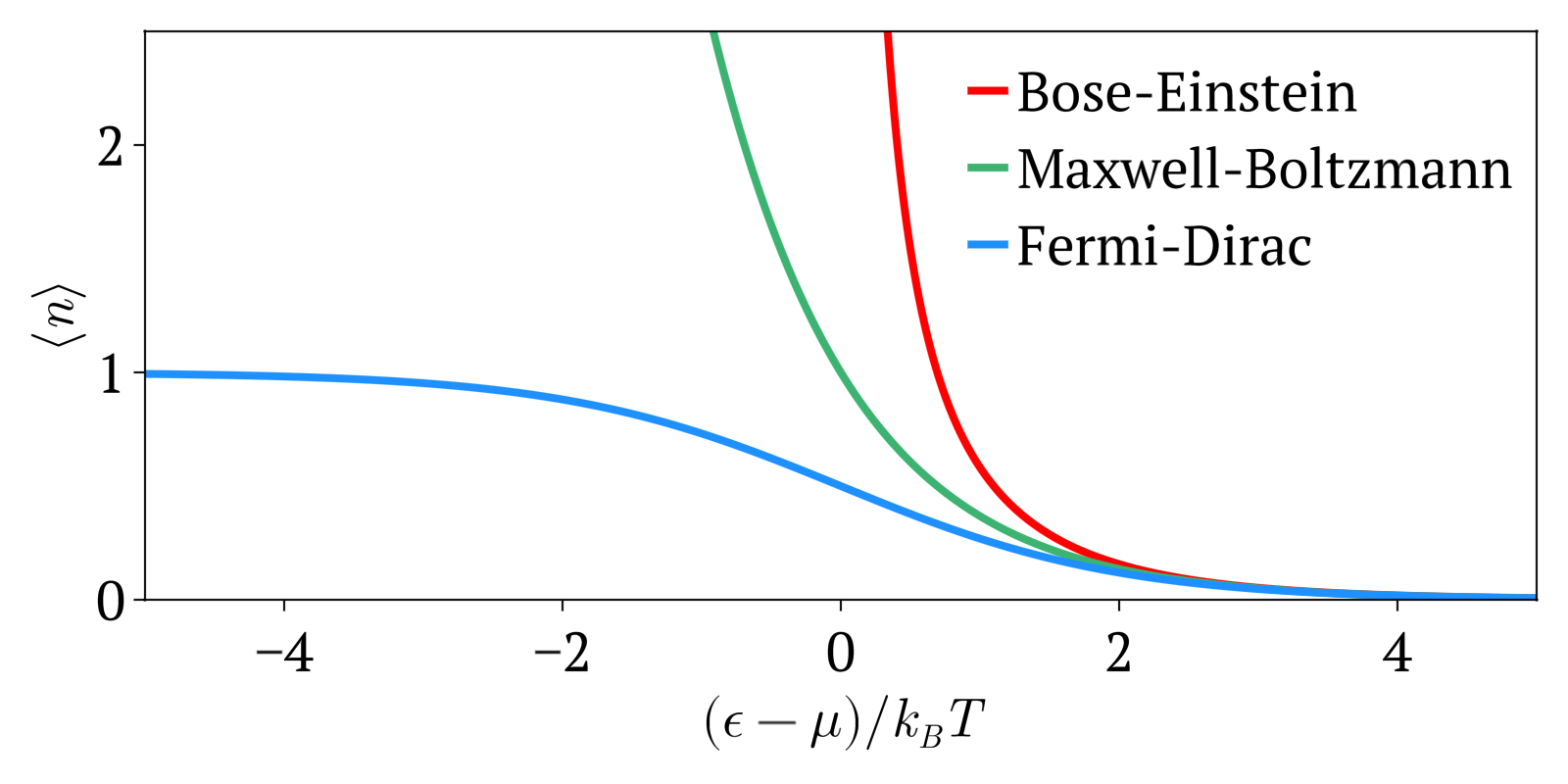
Fermi Dirac Statistics
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian and naturalized American physicist, renowned for being the creator of the world's first artificial nuclear reactor, the Chicago Pile-1, and a member of the Manhattan Project. He has been called the "architect of the nuclear age" and the "architect of the atomic bomb". He was one of very few physicists to excel in both theoretical and experimental physics. Fermi was awarded the 1938 Nobel Prize in Physics for his work on induced radioactivity by neutron bombardment and for the discovery of transuranium elements. With his colleagues, Fermi filed several patents related to the use of nuclear power, all of which were taken over by the US government. He made significant contributions to the development of statistical mechanics, quantum theory, and nuclear and particle physics. Fermi's first major contribution involved the field of statistical mechanics. After Wolfgang Pauli formulated his exclusion principle in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maxwell–Boltzmann Statistics
In statistical mechanics, Maxwell–Boltzmann statistics describes the distribution of classical material particles over various energy states in thermal equilibrium. It is applicable when the temperature is high enough or the particle density is low enough to render quantum effects negligible. The expected number of particles with energy \varepsilon_i for Maxwell–Boltzmann statistics is : \langle N_i \rangle = \frac = \frac\,g_i e^, where: * \varepsilon_i is the energy of the ''i''th energy level, * \langle N_i \rangle is the average number of particles in the set of states with energy \varepsilon_i, * g_i is the degeneracy of energy level ''i'', that is, the number of states with energy \varepsilon_i which may nevertheless be distinguished from each other by some other means,For example, two simple point particles may have the same energy, but different momentum vectors. They may be distinguished from each other on this basis, and the degeneracy will be the number of po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |