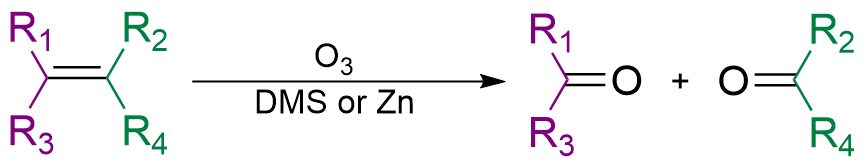|
Dodecanedioic Acid
Dodecanedioic acid (DDDA) is a dicarboxylic acid with the formula . A white solid, the compound finds a variety of applications ranging from polymers to materials. The unbranched compound is the most commonly encountered C12 dicarboxylic acid. Production DDDA has traditionally been produced from butadiene using a multi-step chemical process. Butadiene is first converted to cyclododecatriene through cyclotrimerization. The triene is then hydrogenated to cyclododecane. Autoxidation by air in the presence of boric acid gives a mixture of cyclodecanol and the cyclododecanone. In the final step, this mixture oxidized to the diacid using nitric acid. An alternative route involves ozonolysis of cyclododecene. : Biological process Paraffin wax can be converted into DDDA on a laboratory scale with a special strain of '' Candida tropicalis'' yeast in a multi-step process. Renewable plant-oil feedstocks sourced from switchgrass could also be used to produce DDDA. Uses DDDA os us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarboxylic Acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are used in the preparation of copolymers such as polyamides and polyesters. The most widely used dicarboxylic acid in the industry is adipic acid, which is a precursor in the production of nylon. Other examples of dicarboxylic acids include aspartic acid and glutamic acid, two amino acids in the human body. The name can be abbreviated to diacid. Linear saturated dicarboxylic acids The general formula is .Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. The PubChem links gives access to more information on the compounds, including other names, ids, toxicity and saf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraffin Wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and begins to melt above approximately , and its boiling point is above . Common applications for paraffin wax include lubrication, electrical insulation, and candles; dyed paraffin wax can be made into crayons. It is distinct from kerosene and other petroleum products that are sometimes called paraffin. Un-dyed, unscented paraffin candles are odorless and bluish-white. Paraffin wax was first created by Carl Reichenbach in Germany in 1830 and marked a major advancement in candlemaking technology, as it burned more cleanly and reliably than tallow candles and was cheaper to produce. In chemistry, ''paraffin'' is used synonymously with ''alkane'', indicating hydrocarbons with the general formula C''n''H2''n''+2. The name is derived from Latin ''par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic. Nylon is a silk-like thermoplastic, generally made from petroleum, that can be melt-processed into fibers, films, or shapes. Nylon polymers can be mixed with a wide variety of additives to achieve many property variations. Nylon polymers have found significant commercial applications in fabric and fibers (apparel, flooring and rubber reinforcement), in shapes (molded parts for cars, electrical equipment, etc.), and in films (mostly for food packaging). History DuPont and the invention of nylon Researchers at DuPont began developing cellulose based fibers, culminating in the synthetic fiber rayon. DuPont's experience with rayon was an important precursor to its development and marketing of nylon. DuPont's invention of nylon spanned an eleven-year period, ranging from the initial researc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Engineering Plastic
Engineering plastics are a group of plastic materials that have better mechanical and/or thermal properties than the more widely used commodity plastics (such as polystyrene, PVC, polypropylene and polyethylene). Being more expensive than standard plastics, engineering plastics are produced in lower quantities and tend to be used for smaller objects or low-volume applications (such as mechanical parts), rather than for bulk and high-volume ends (like containers and packaging). Engineering plastics have a higher heat resistance than standard plastics and are continuously usable at temperatures up to about 150 °C. The term usually refers to thermoplastic materials rather than thermosetting ones. Examples of engineering plastics include polyamides (PA, nylons), used for skis and ski boots; polycarbonates (PC), used in motorcycle helmets and optical discs; and poly(methyl methacrylate) (PMMA, major brand names acrylic glass and plexiglass), used e.g. for taillights and protect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'', mean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiseptics
An antiseptic (from Greek ἀντί ''anti'', "against" and σηπτικός ''sēptikos'', "putrefactive") is an antimicrobial substance or compound that is applied to living tissue/skin to reduce the possibility of infection, sepsis, or putrefaction. Antiseptics are generally distinguished from ''antibiotics'' by the latter's ability to safely destroy bacteria within the body, and from ''disinfectants'', which destroy microorganisms found on non-living objects. Antibacterials include antiseptics that have the proven ability to act against bacteria. Microbicides which destroy virus particles are called viricides or antivirals. Antifungals, also known as antimycotics, are pharmaceutical fungicides used to treat and prevent mycosis (fungal infection). Surgery The widespread introduction of antiseptic surgical methods was initiated by the publishing of the paper '' Antiseptic Principle of the Practice of Surgery'' in 1867 by Joseph Lister, which was inspired by Louis Pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Switchgrass
''Panicum virgatum'', commonly known as switchgrass, is a perennial warm season bunchgrass native to North America, where it occurs naturally from 55°N latitude in Canada southwards into the United States and Mexico. Switchgrass is one of the dominant species of the central North American tallgrass prairie and can be found in remnant prairies, in native grass pastures, and naturalized along roadsides. It is used primarily for soil conservation, forage production, game cover, as an ornamental grass, in phytoremediation projects, fiber, electricity, heat production, for biosequestration of atmospheric carbon dioxide, and more recently as a biomass crop for ethanol and butanol. Other common names for switchgrass include tall panic grass, Wobsqua grass, blackbent, tall prairiegrass, wild redtop, thatchgrass, and Virginia switchgrass. Description Switchgrass is a hardy, deep-rooted, perennial rhizomatous grass that begins growth in late spring. It can grow up to high, but is ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Candida Tropicalis
''Candida tropicalis'' is a species of yeast in the genus ''Candida (fungus), Candida''. It is a common pathogen in neutropenic hosts, in whom it may spread through the bloodstream to peripheral organs. For invasive disease, treatments include amphotericin B, echinocandins, or extended-spectrum triazole antifungals. History and taxonomy In the history of fungi, the name of genus ''Candida'', derived from the family Debaryomycetaceae, comes from the Latin term "" which has the meaning of “glowing white” and also refers to as smooth and glistering. Genus ''Candida'' referred to any asexual yeast without any of the following characteristics: production of acetic acid, pigments of colours red, pink or orange, arthroconidia, unipolar or bipolar budding, enteroblastic-basipetal budding, blastoconidia formation on sympodulae, buds formation on stalks, triangular cells, needle-shaped terminal conidia, and having the ability to grow on inositol as a sole carbon source. Although there a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl () group while azo compounds form nitrosamines (). The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions. Ozonolysis of alkenes Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates complete consumption of the alkene. Alternatively, various other chemicals can be used as indicators of this endpoint by detecting the presence of ozone. If ozonolysis is performed by bubbling a stream of ozone-enriched oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |