|
Diquat
Diquat is the International Organization for Standardization, ISO trivial name, common name for an Ion#Anions and cations, organic dication that, as a Salt (chemistry), salt with counterions such as bromide or chloride is used as a contact herbicide that produces desiccation and defoliation. Diquat is no longer approved for use in the European Union, although its Pesticide#Regulation, registration in many other countries including the USA is still valid. Synthesis Pyridine is oxidatively coupled to form 2,2'-Bipyridine, 2,2′-bipyridine over a heated Raney nickel catalyst. The ethylene bridge is formed by the reaction with 1,2-dibromoethane History Diquat's herbicidal properties were recognized in 1955 in the Imperial Chemical Industries (ICI) laboratories at Jealott's Hill, following its first synthesis at ICI's Dyestuffs Division in Blackley, England. It was active on test plants at application rates as low as 0.1 lb/acre. It was found that only those quaternary salts which wer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diquat Synthesis V1
Diquat is the ISO common name for an organic dication that, as a salt with counterions such as bromide or chloride is used as a contact herbicide that produces desiccation and defoliation. Diquat is no longer approved for use in the European Union, although its registration in many other countries including the USA is still valid. Synthesis Pyridine is oxidatively coupled to form 2,2′-bipyridine over a heated Raney nickel catalyst. The ethylene bridge is formed by the reaction with 1,2-dibromoethane History Diquat's herbicidal properties were recognized in 1955 in the Imperial Chemical Industries (ICI) laboratories at Jealott's Hill, following its first synthesis at ICI's Dyestuffs Division in Blackley, England. It was active on test plants at application rates as low as 0.1 lb/acre. It was found that only those quaternary salts which were capable of being converted by reducing agents to radical cations had herbicidal activity and another of these was paraquat, which was more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated Polymer, polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties Pyridine is diamagnetism, diamagnetic. Its critical point (thermodynamics), critical parameters are: pressure 5.63 MPa, temperature 619 K and volume 248 cm3/mol. In the temperatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbicide
Herbicides (, ), also commonly known as weed killers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page for EPA reports on pesticide use ihere Selective herbicides control specific weed species while leaving the desired crop relatively unharmed, while non-selective herbicides (sometimes called "total weed killers") kill plants indiscriminately. The combined effects of herbicides, nitrogen fertilizer, and improved cultivars has increased yields (per acre) of major crops by three to six times from 1900 to 2000. In the United States in 2012, about 91% of all herbicide usage, was determined by weight applied, in agriculture. In 2012, world pesticide expenditures totaled nearly US$24.7 billion; herbicides were about 44% of those sales and constituted the biggest portion, followed by insecticides, fungicides, and fumigants. Herbicide is also used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraquat
Paraquat ( trivial name; ), or ''N'',''N''′-dimethyl-4,4′-bipyridinium dichloride (systematic name), also known as methyl viologen, is a toxic organic compound with the chemical formula C6H7N)2l2. It is classified as a viologen, a family of redox-active heterocycles of similar structure. It is one of the most widely used herbicides worldwide. It is quick-acting and non-selective, killing green plant tissue on contact. Paraquat is highly toxic to humans and other animals. The toxicity and lethality depends on the dose and how the herbicide is absorbed by the body. In humans, paraquat damages the mouth, stomach, and intestines if it is ingested orally. Once absorbed in the body, paraquat causes particular damage to the lungs, kidneys, and liver. Paraquat's lethality is attributed to its enhancing production of superoxide anions and human lung cells can accumulate paraquat. Paraquat exposure has been strongly linked to the development of Parkinson's disease. Paraquat may be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jealott's Hill
Jealott's Hill is a village in the county of Berkshire, England, within the civil parish of Warfield. The settlement is on the A3095 road approximately north of Bracknell. The nearest railway station is in . History The name of the hill is reported to have derived from the surname of a 14th-century landowner, Roger Jolyl. This name evolved into "Joyliff's Hill" and then, on Henry Walter's ''Map of Windsor Forest, 1823'', became "Jealous Hill". This changed again to "Jealot's Hill" on John Snare's 1846 map and by the 1920s the modern spelling was established. Syngenta research site Jealott's Hill is home to Syngenta's largest research and development site which includes a large agricultural research greenhouse at and a farm. , Syngenta employed around 800 people there. The site was formed in 1927 by the amalgamation of three farms, Hawthorndale, Nuptown and Jealott's Hill itself. Jealott's Hill House was built in 1928 and officially opened on 28 June 1929 as the offices, lab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Federal Insecticide, Fungicide, And Rodenticide Act
The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) is a United States federal law that set up the basic U.S. system of pesticide regulation to protect applicators, consumers, and the environment. It is administered and regulated by the United States Environmental Protection Agency (EPA) and the appropriate environmental agencies of the respective states. FIFRA has undergone several important amendments since its inception. A significant revision in 1972 by the Federal Environmental Pesticide Control Act (FEPCA) and several others have expanded EPA's present authority to oversee the sales and use of pesticides with emphasis on the preservation of human health and protection of the environment by "(1) strengthening the registration process by shifting the burden of proof to the chemical manufacturer, (2) enforcing compliance against banned and unregistered products, and (3) promulgating the regulatory framework missing from the original law". History The Federal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Environmental Protection Agency
The Environmental Protection Agency (EPA) is an independent agency of the United States government tasked with environmental protection matters. President Richard Nixon proposed the establishment of EPA on July 9, 1970; it began operation on December 2, 1970, after Nixon signed an executive order. The order establishing the EPA was ratified by committee hearings in the House and Senate. The agency is led by its administrator, who is appointed by the president and approved by the Senate. The current administrator is Lee Zeldin. The EPA is not a Cabinet department, but the administrator is normally given cabinet rank. The EPA has its headquarters in Washington, D.C. There are regional offices for each of the agency's ten regions, as well as 27 laboratories around the country. The agency conducts environmental assessment, research, and education. It has the responsibility of maintaining and enforcing national standards under a variety of environmental laws, in consultat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pesticide Regulation In The United States
Pesticide regulation in the United States is primarily a responsibility of the Environmental Protection Agency (EPA). In America, it was not till the 1950s that pesticides were regulated in terms of their safety. The Pesticides Control Amendment (PCA) of 1954 was the first time Congress passed guidance regarding the establishment of safe limits for pesticide residues on food. It authorized the Food and Drug Administration (FDA) to ban pesticides they determined to be unsafe if they were sprayed directly on food. The Food Additives Amendment, which included the Delaney Clause, prohibited the pesticide residues from any carcinogenic pesticides in processed food. In 1959, pesticides were required to be registered. In 1970, President Richard Nixon created the EPA and shifted control of pesticide regulation from the US Department of Agriculture (USDA), the US Department of the Interior (DOI), and FDA to the newly created agency. By this time, public awareness of potential human hea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
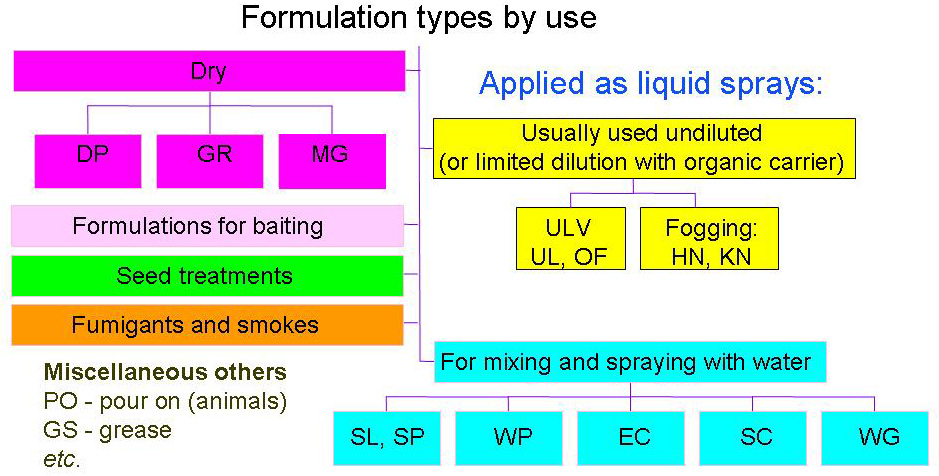
Pesticide Formulation
The biological activity of a pesticide, be it chemical or biological in nature, is determined by its active ingredient (AI - also called the ''active substance''). Pesticide products very rarely consist of the pure active ingredient. The AI is usually formulated with other materials (adjuvents and co-formulants) and this is the product as sold, but it may be further diluted in use. Formulations improve the properties of a chemical for handling, storage, application and may substantially influence effectiveness and safety. Formulation terminology follows a 2-letter convention: (''e.g.'' GR: granules) listed by CropLife International (formerly GIFAP then GCPF) in the ''Catalogue of Pesticide Formulation Types'' (Monograph 2); see download page Some manufacturers do not follow these industry standards, which can cause confusion for users. Water-miscible formulations By far the most frequently used products are formulations for mixing with water then applying as sprays. Water miscibl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl radical (OH.), and singlet oxygen(1O2). ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations. Inventory of ROS ROS are not uniformly defined. All sources include superoxide, singlet oxygen, and hydroxyl radical. Hydrogen peroxide is not nearly as reactive as these s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic organism, anaerobic bacterium ''Clostridium pasteurianum''. Another redox protein, isolated from spinach chloroplasts, was termed "chloroplast ferredoxin". The chloroplast ferredoxin is involved in both cyclic and non-cyclic photophosphorylation reactions of photosynthesis. In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor thus reducing the enzyme NADP+ reductase. It accepts electrons produced from sunlight-Electron excitation, excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase . Ferredoxins are small proteins containing iron and sulfur atoms organized as iron–sulfur clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosystem I
Photosystem I (PSI, or plastocyanin–ferredoxin oxidoreductase) is one of two photosystems in the Light-dependent reactions, photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane protein Protein complex, complex that uses light energy to catalyze the electron transfer, transfer of electrons across the thylakoid membrane from plastocyanin to ferredoxin. Ultimately, the electrons that are transferred by Photosystem I are used to produce the moderate-energy hydrogen carrier NADPH. The photon energy absorbed by Photosystem I also produces a Chemiosmosis, proton-motive force that is used to generate adenosine triphosphate, ATP. PSI is composed of more than 110 Cofactor (biochemistry), cofactors, significantly more than Photosystem II. History This photosystem is known as PSI because it was discovered before Photosystem II, although future experiments showed that Photosystem II is actually the first enzyme of the phot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |