|
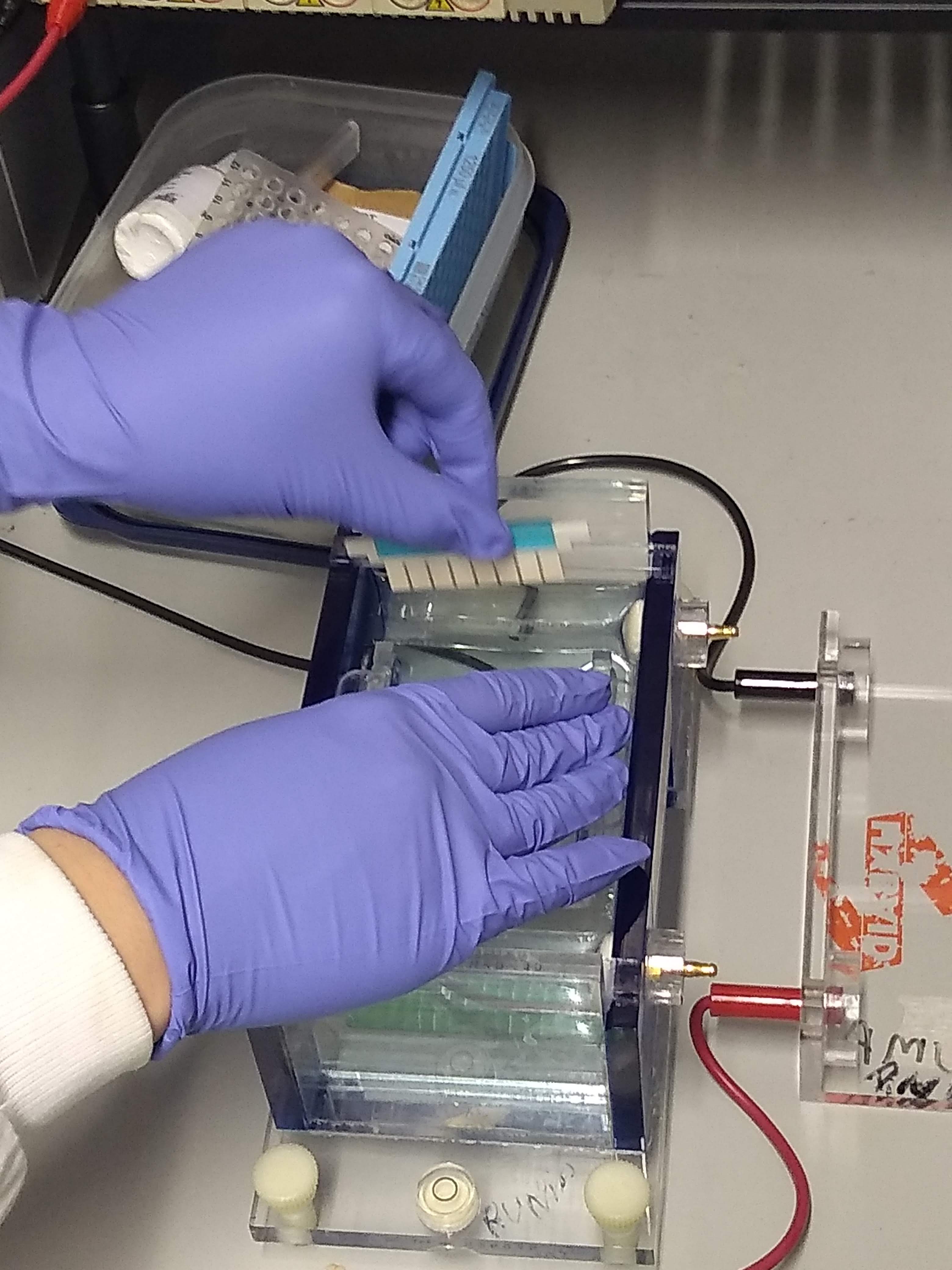
Difference Gel Electrophoresis
Difference gel electrophoresis (DIGE) is a form of gel electrophoresis where up to three different protein samples can be labeled with size-matched, charge-matched spectrally resolvable fluorescent dyes (for example Cy3, Cy5, Cy2) prior to two dimensional gel electrophoresis.Unlü M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997 Oct;18(11):2071-7. PMI9420172 Procedure The three samples are mixed and loaded onto IEF (isoelectric focusing chromatography) for first dimension and the strip is transferred to a SDS PAGE. After the gel electrophoresis, the gel is scanned with the excitation wavelength of each dye one after the other, so each sample can be seen separately (if we scan the gel at the excitation wavelength of the Cy3 dye, we will see in the gel only the sample that was labeled with that dye). This technique is used to see changes in protein abundance (for example, between a sample of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gel Electrophoresis
Gel electrophoresis is an electrophoresis method for separation and analysis of biomacromolecules (DNA, RNA, proteins, etc.) and their fragments, based on their size and charge through a gel. It is used in clinical chemistry to separate proteins by charge or size (IEF agarose, essentially size independent) and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments, or to separate proteins by charge. Nucleic acid molecules are separated by applying an electric field to move the negatively charged molecules through a gel matrix of agarose, polyacrylamide, or other substances. Shorter molecules move faster and migrate farther than longer ones because shorter molecules migrate more easily through the pores of the gel. This phenomenon is called sieving. Proteins are separated by the charge in agarose because the pores of the gel are too large to sieve proteins. Gel electrophoresi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Dye
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescence, fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromaticity, aromatic groups, or planar or cyclic molecules with several pi bond, π bonds. Fluorophores are sometimes used alone, as a dye tracing, tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalent bond, covalently bonded to macromolecules, serving as a markers (or dyes, or tags, or reporters) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, such as Fluorescence microscope, fluorescent imaging and fluorescence spectroscopy, spectroscopy. Fluorescein, via its amine-react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Two Dimensional Gel Electrophoresis
2 (two) is a number, numeral and digit. It is the natural number following 1 and preceding 3. It is the smallest and the only even prime number. Because it forms the basis of a duality, it has religious and spiritual significance in many cultures. Mathematics The number 2 is the second natural number after 1. Each natural number, including 2, is constructed by succession, that is, by adding 1 to the previous natural number. 2 is the smallest and the only even prime number, and the first Ramanujan prime. It is also the first superior highly composite number, and the first colossally abundant number. An integer is determined to be even if it is divisible by two. When written in base 10, all multiples of 2 will end in 0, 2, 4, 6, or 8; more generally, in any even base, even numbers will end with an even digit. A digon is a polygon with two sides (or edges) and two vertices. Two distinct points in a plane are always sufficient to define a unique line in a nontrivi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoelectric Focusing
Isoelectric focusing (IEF), also known as electrofocusing, is a technique for separating different charged molecules by differences in their isoelectric point (pI). It is a type of zone electrophoresis usually performed on proteins in a gel that takes advantage of the fact that overall charge on the molecule of interest, i.e. the net charge density, is a function of the pH of its surroundings. Procedure IEF involves adding an ampholyte solution into immobilized pH gradient (IPG) gels. IPGs are the acrylamide gel matrix co-polymerized with the pH gradient, which result in completely stable gradients except the most alkaline (>12) pH values. The immobilized pH gradient is obtained by the continuous change in the ratio of ''immobilines''. An immobiline is a weak acid or base defined by its pK value. A protein that is in a pH region below its isoelectric point (pI) will be positively charged and so will migrate toward the cathode (negatively charged electrode). As it migrates th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excitation Wavelength
Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum. Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet–visible spectroscopy are particularly common in analytical applications. Absorption spectroscopy is also employed in studies of molecular and atomic physics, astronomical spectroscopy and remote sensing. There is a wide range of experimental approaches for measuring absorption spectra. The most common ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoelectric Point
The isoelectric point (pI, pH(I), IEP), is the pH at which a molecule carries no net electric charge, electrical charge or is electrically neutral in the statistical mean. The standard nomenclature to represent the isoelectric point is pH(I). However, pI is also used. For concision, brevity, this article uses pI. The net charge on the molecule is affected by pH of its surrounding environment and can become more positively or negatively charged due to the gain or loss, respectively, of protons#In Physics and biochemistry, protons (H+). Surfaces naturally charge to form a double layer (interfacial), double layer. In the common case when the surface charge-determining ions are H+/HO−, the net surface charge is affected by the pH of the liquid in which the solid is submerged. The pI value can affect the solubility of a molecule at a given pH. Such molecules have minimum solubility in water or salt solutions at the pH that corresponds to their pI and often precipitate out of Solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protomap (proteomics)
Benjamin Franklin Cravatt III is a professor in the Department of Chemistry at The Scripps Research Institute in La Jolla, California. Considered a co-inventor of activity-based proteomics and a substantial contributor to research on the endocannabinoid system, he is a prominent figure in the field of chemical biology. Cravatt was elected to the National Academy of Sciences in 2014, and the American Academy of Arts and Sciences in 2016. He is Gilula Chair of Chemical Biology, a Cope Scholar, and a Searle Scholar. Early life and education His father was a dentist and his mother a dental hygienist, both of whom instilled in Cravatt an interest in biology as a child. Ben Cravatt is left handed. Cravatt entered Stanford University in 1988, graduating in 1992 with a BS in the Biological Sciences and a BA in History. He then received a PhD in Macromolecular and Cellular Structure and Chemistry from The Scripps Research Institute in 1996, where he worked under the joint supervis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |