|
Dibutylchloromethyltin Chloride
Dibutylchloromethyltin chloride (DBCT) is a toxic organotin compound. It is a potent and irreversible ATP synthase inhibitor. DBCT is a volatile liquid with powerful vesicant effects. See also *Tributyltin chloride *Oligomycin Oligomycins are macrolides created by ''Streptomyces'' that are strong antibacterial agents but are often poisonous to other organisms, including humans. Function Oligomycins have use as antibiotics. However, in humans, they have limited or n ... References ATP synthase inhibitors Organotin compounds Organochlorides Blister agents Tin(IV) compounds Chloromethyl compounds {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vesicant
A blister agent (or vesicant) is a chemical compound that causes severe skin, eye and mucosal pain and irritation in the form of severe chemical burns resulting in fluid filled blisters. Named for their ability to cause vesication, blister agent refers in common parlance to those agents which are developed for, or have been in the past utilized as chemical weapons, though some naturally occurring substances such as cantharidin fall under the same terminology.Cantharidin and Meloids: a review of classical history, biosynthesis, and function Exposure to blister agents is widely incapacitating, but often precipitates a delayed effect, with symptoms developing one to twenty four hours following the initial contact with the agent. Tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compound
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known, but are less importan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
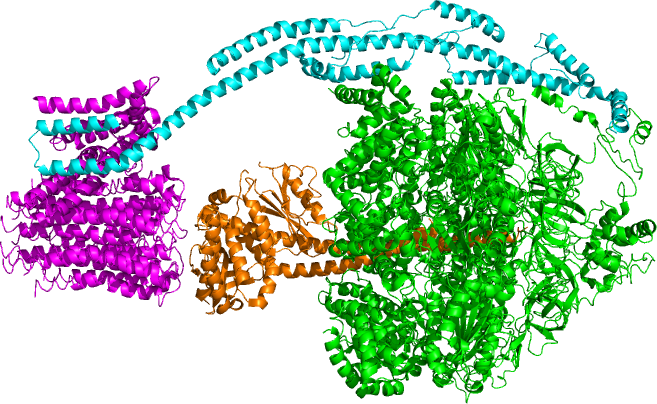
ATP Synthase
ATP synthase is an enzyme that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is: * ADP + Pi + 2H+out ATP + H2O + 2H+in ATP synthase lies across a cellular membrane and forms an aperture that hydron (chemistry), protons can cross from areas of high concentration to areas of low concentration, imparting energy for the synthesis of ATP. This electrochemical gradient is generated by the electron transport chain and allows cells to store energy in ATP for later use. In prokaryote, prokaryotic cells ATP synthase lies across the plasma membrane, while in eukaryote, eukaryotic cells it lies across the inner mitochondrial membrane. Organisms capable of photosynthesis also have ATP synthase across the thylakoid membrane, which in plants is located in the chloroplast and in cyanobacteria is located in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tributyltin Chloride
Tributyltin chloride is an organotin compound with the formula ( C4H9)3SnCl. It is a colorless liquid that is soluble in organic solvents. Preparation and reactions The compound is prepared by a redistribution reaction by combining stannic chloride and tetrabutyltin: :3 (C4H9)4Sn + SnCl4 → 4 (C4H9)3SnCl Tributyltin chloride hydrolyzes to the oxide C4H9)3Snsub>2O Tributyltin chloride is used as a precursor to other organotin compounds{{cite journal, title=Palladium-catalyzed Coupling Of Acid Chlorides With Organotin Reagents: Ethyl (E)-4-(4-nitrophenyl)-4-oxo-2-butenoate, author=A. F. Renaldo , author2=J. W. Labadie , author3=J. K. Stille , journal=Org. Synth., year=1989, volume=67, page=86, doi=10.15227/orgsyn.067.0086 and reagents, such as tributyltin hydride Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligomycin
Oligomycins are macrolides created by ''Streptomyces'' that are strong antibacterial agents but are often poisonous to other organisms, including humans. Function Oligomycins have use as antibiotics. However, in humans, they have limited or no clinical use due to their toxic effects on mitochondria and ATP synthase. Oligomycin A is an inhibitor of ATP synthase. In oxidative phosphorylation research, it is used to prevent stage 3 (phosphorylating) respiration. Oligomycin A inhibits ATP synthase by blocking its proton channel (FO subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production). The inhibition of ATP synthesis by oligomycin A will significantly reduce electron flow through the electron transport chain; however, electron flow is not stopped completely due to a process known as ''proton leak'' or '' mitochondrial uncoupling''. This process is due to facilitated diffusion of protons into the mitochondrial matrix through an uncoupling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATP Synthase Inhibitors
ATP may refer to: Science, technology and biology *Adenosine triphosphate, an organic chemical used for driving biological processes **ATPase, any enzyme that makes use of adenosine triphosphate *Advanced Technology Program, US government program *Alberta Taciuk process, for extracting oil from shale, etc. *Anti-tachycardia pacing, process similar to a pacemaker * Assistive Technology Practitioner - Rehabilitation Engineering and Assistive Technology Society of North America (RESNA) *AT Protocol, an open communications protocol intended for decentralized social networking services *Automated theorem proving, method of proving mathematical theorems by computer programs Companies and organizations * Association of Tennis Professionals, men's professional tennis governing body **ATP Tour * American Technical Publishers, employee-owned publishing company * Armenia Tree Project, non-profit organization * Association for Transpersonal Psychology * ATP architects engineers, archite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compounds
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known, but are less imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochlorides
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds that contain one or more carbon–chlorine bonds. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Organochlorides such as trichloroethylene, tetrachloroethylene, dichloromethane and chloroform are commonly used as solvents and are referred to as "chlorinated solvents". Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. They have hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blister Agents
A blister agent (or vesicant) is a chemical compound that causes severe skin, eye and mucosal pain and irritation in the form of severe Chemical burn, chemical burns resulting in fluid filled blisters. Named for their ability to cause Blister, vesication, blister agent refers in common parlance to those agents which are developed for, or have been in the past utilized as Chemical weapon, chemical weapons, though some naturally occurring substances such as cantharidin fall under the same terminology.Cantharidin and Meloids: a review of classical history, biosynthesis, and function Exposure to blister agents is widely incapacitating, but often precipitates a delayed effect, with symptoms developing one to twenty four hours following t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(IV) Compounds
Tin is a chemical element; it has chemical symbol, symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the so-called "tin cry", as a result of crystal twinning, twinning in tin crystals. Tin is a post-transition metal in Carbon group, group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains tin(IV) oxide, stannic oxide, . Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundance of the chemical elements, abundant element on Earth, making up 0.00022% of its crust, and with 10 stable isotopes, it has the largest number of stable isotopes in the periodic table, due to its magic number (physics), magic number of protons. It has two main allotrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |