|
Deuruxolitinib
Deuruxolitinib, sold under the brand name Leqselvi, is a medication used for the treatment of alopecia areata. It is a Janus kinase inhibitor selective for JAK1 and JAK2. Although the relative effectiveness of deuruxolitinib and another Janus kinase inhibitor—baricitinib—for alopecia areata may vary depending on the population studied, both drugs are more effective than alternative treatments. Deuruxolitinib was approved for medical use in the United States in July 2024. Medical uses Deuruxolitinib is indicated for the treatment of adults with severe alopecia areata. Side effects The FDA prescribing label for deuruxolitinib contains a boxed warning for serious infections; malignancies; cardiovascular death, myocardial infarction, and stroke; and thrombosis. Society and culture Legal status Deuruxolitinib was approved for medical use in the United States in July 2024. Names Deuruxolitinib is the international nonproprietary name and the United States Adopted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Janus Kinase Inhibitor
A Janus kinase inhibitor, also known as JAK inhibitor or jakinib, is a type of immune modulating medication, which inhibits the activity of one or more of the Janus kinase family of enzymes ( JAK1, JAK2, JAK3, TYK2), thereby interfering with the JAK-STAT signaling pathway in lymphocytes. JAK inhibitors are used in the treatment of some cancers and inflammatory diseases such as rheumatoid arthritis and various skin conditions. A Janus kinase 3 inhibitor is attractive as a possible treatment of various autoimmune diseases since its function is mainly restricted to lymphocytes. JAK inhibitors can suppress the signaling of pro- inflammatory cytokines. Pro-inflammatory cytokines are major contributors to the cause of an over active immune system, resulting in inflammation and pain. JAK inhibitors have the ability to slow down this over activity by the suppression of the intracellular signaling. Contraindications JAK enzymes are part of the JAK/STAT pathway. This signaling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
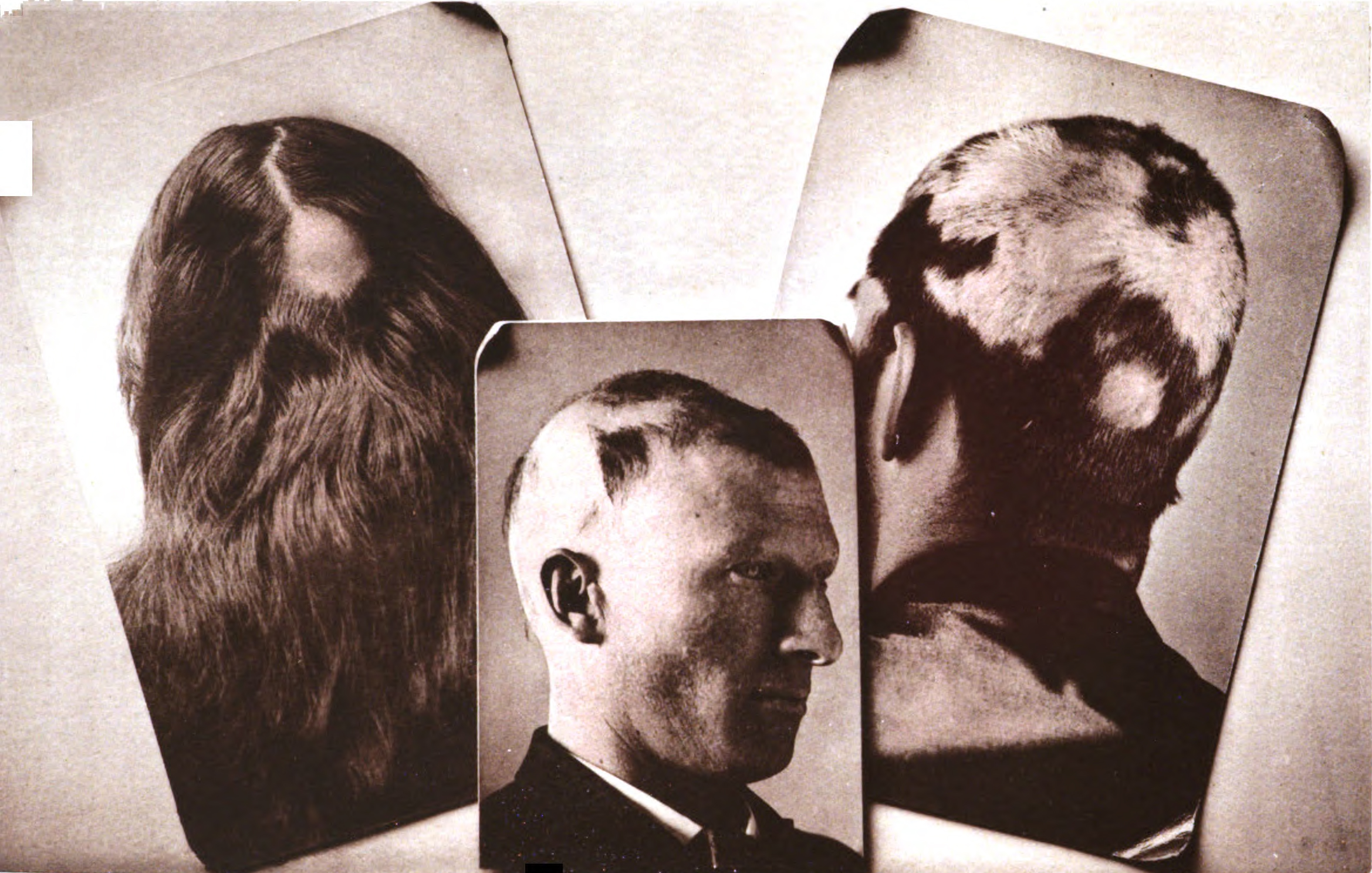
Alopecia Areata
Alopecia areata (AA), also known as spot baldness, is a condition in which hair loss, hair is lost from some or all areas of the body. It often results in a few Baldness, bald spots on the scalp, each about the size of a coin. Psychological stress and illness are possible factors in bringing on alopecia areata in individuals at risk, but in most cases there is no obvious trigger. People are generally otherwise healthy. In a few cases, all the hair on the scalp is lost (''alopecia totalis''), or all body hair is lost (''alopecia universalis''). Hair loss can be permanent or temporary. Alopecia areata is believed to be an autoimmune disease resulting from a breach in the immune privilege of the hair follicles. Risk factors include a family history of the condition. Among identical twins, if one is affected, the other has about a 50% chance of also being affected. The underlying mechanism involves failure by the body to recognize its own cells, with subsequent immune-mediated des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
By Mouth
Oral administration is a route of administration whereby a substance is taken through the Human mouth, mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications. Oral administration can be easier and less painful than other routes of administration, such as Injection (medicine), injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth". The expression is used in medicine to describe a treatment that is taken orally (but not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JAK2
Janus kinase 2 (commonly called JAK2) is a non-receptor tyrosine kinase. It is a member of the Janus kinase family and has been implicated in signaling by members of the type II cytokine receptor family (e.g. interferon receptors), the GM-CSF receptor family ( IL-3R, IL-5R and GM-CSF-R), the gp130 receptor family (e.g., IL-6R), and the single chain receptors (e.g. Epo-R, Tpo-R, GH-R, PRL-R). The distinguishing feature between janus kinase 2 and other JAK kinases is the lack of Src homology binding domains ( SH2/ SH3) and the presence of up to seven JAK homology domains (JH1-JH7). Nonetheless the terminal JH domains retain a high level of homology to tyrosine kinase domains. An interesting note is that only one of these carboxy-terminal JH domains retains full kinase function (JH1) while the other (JH2), previously thought to have no kinase functionality and accordingly termed a pseudokinase domain, has since been found to be catalytically active, albeit at only 10% t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baricitinib
Baricitinib, sold under the brand name Olumiant among others, is an immunomodulatory medication used for the treatment of rheumatoid arthritis, alopecia areata, and COVID-19. It acts as an inhibitor of janus kinase (JAK), blocking the subtypes JAK1 and JAK2. Baricitinib is approved for medical use in the European Union Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. and in the United States. Medical uses In February 2017, baricitinib was approved for use in the European Union as a second-line therapy for moderate to severe active rheumatoid arthritis in adults, either alone or in combination with methotrexate. In May 2018, the US Food and Drug Administration (FDA) approved baricitinib for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more TNF antagonist therapies. In May 2022, the FDA approved ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indicated
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis is the assessment that a particular medical condition is present while an indication is a reason for use. The opposite of an indication is a contraindication, a reason to withhold a certain medical treatment because the risks of treatment clearly outweigh the benefits. In the United States, indications for prescription drugs are approved by the FDA. Indications are included in the Indications and Usage section of the Prescribing Information. The primary role of this section of labeling is to enable health care practitioners to readily identify appropriate therapies for patients by clearly communicating the drug's approved indication(s). The Indications and Usage section states the disease or condition, or manifestation or symptoms thereof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boxed Warning
In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears near the beginning of the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a "box" or border around the text to emphasize its importance. The FDA can require a pharmaceutical company to place a boxed warning. It is the strongest warning that the FDA requires, and signifies that medical studies indicate that the drug carries a significant risk of preventable, serious or even life-threatening adverse effects. Economists and physicians have thoroughly studied the effects of FDA boxed warnings on prescription patterns. It is not necessarily true that a physician and patient will have a conversation about a drug's boxed warning after it is issued. For instance, an FDA-mandated boxed warning decreased rosiglitazone use by 70%, but that still meant 3.8 million people were given th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Adopted Name
A United States Adopted Name (USAN) is a unique nonproprietary name assigned to a medication marketed in the United States. Each name is assigned by the USAN Council, which is co-sponsored by the American Medical Association (AMA), the United States Pharmacopeial Convention (USP), and the American Pharmacists Association (APhA). The USAN Program states that its goal is to select simple, informative, and unique nonproprietary names (also called generic names) for drugs by establishing logical nomenclature classifications based on pharmacological or chemical relationships. In addition to drugs, the USAN Council names agents for gene therapy and cell therapy, contact lens polymers, surgical materials, diagnostics, carriers, and substances used as an excipient. The USAN Council works in conjunction with the World Health Organization (WHO) international nonproprietary name (INN) Expert Committee and national nomenclature groups to standardize drug nomenclature and establish rule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Janus Kinase Inhibitors
In ancient Roman religion and myth, Janus ( ; ) is the god of beginnings, gates, transitions, time, duality, doorways, passages, frames, and endings. He is usually depicted as having two faces. The month of January is named for Janus (''Ianuarius''). According to ancient Roman farmers' almanacs, Juno was mistaken as the tutelary deity of the month of January, but Juno is the tutelary deity of the month of June. Janus presided over the beginning and ending of conflict, and hence war and peace. The gates of the Temple of Janus in Rome were opened in time of war and closed to mark the arrival of peace. As a god of transitions, he had functions pertaining to birth and to journeys and exchange, and in his association with Portunus, a similar harbor and gateway god, he was concerned with travelling, trading, and shipping. Janus had no flamen or specialised priest ''( sacerdos)'' assigned to him, but the King of the Sacred Rites ''(rex sacrorum)'' himself carried out his ceremoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |