|
Delocalisation
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref> The term delocalization is general and can have slightly different meanings in different fields: * In organic chemistry, it refers to resonance in conjugated systems and aromatic compounds. * In solid-state physics, it refers to free electrons that facilitate electrical conduction. * In quantum chemistry, it refers to molecular orbital electrons that have extended over several adjacent atoms. Resonance In the simple aromatic ring of benzene, the delocalization of six π electrons over the C6 ring is often graphically indicated by a circle. The fact that the six C-C bonds are equidistant is one indication that the electrons are delocalized; if the structure were to have isolated double bonds alternating with discrete single bonds, the bond would likewise have alternating longer and shorter l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Ring Current
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field. The ring current creates its own magnetic field. Outside the ring, this field is in the same direction as the externally applied magnetic field; inside the ring, the field counteracts the externally applied field. As a result, the net magnetic field outside the ring is greater than the externally applied field alone, and is less inside the ring. Relevance to NMR spectroscopy Aromatic ring currents are relevant to NMR spectroscopy, as they dramatically influence the chemical shift ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unitary Transformation
In mathematics, a unitary transformation is a linear isomorphism that preserves the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation. Formal definition More precisely, a unitary transformation is an isometric isomorphism between two inner product spaces (such as Hilbert spaces). In other words, a ''unitary transformation'' is a bijective function :U : H_1 \to H_2 between two inner product spaces, H_1 and H_2, such that :\langle Ux, Uy \rangle_ = \langle x, y \rangle_ \quad \text x, y \in H_1. It is a linear isometry, as one can see by setting x=y. Unitary operator In the case when H_1 and H_2 are the same space, a unitary transformation is an automorphism of that Hilbert space, and then it is also called a unitary operator. Antiunitary transformation A closely related notion is that of antiunitary transformation, which is a bijective function :U:H_1\to H_2\, between two complex Hilbert spaces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Combination
In mathematics, a linear combination or superposition is an Expression (mathematics), expression constructed from a Set (mathematics), set of terms by multiplying each term by a constant and adding the results (e.g. a linear combination of ''x'' and ''y'' would be any expression of the form ''ax'' + ''by'', where ''a'' and ''b'' are constants). The concept of linear combinations is central to linear algebra and related fields of mathematics. Most of this article deals with linear combinations in the context of a vector space over a field (mathematics), field, with some generalizations given at the end of the article. Definition Let ''V'' be a vector space over the field ''K''. As usual, we call elements of ''V'' ''vector space, vectors'' and call elements of ''K'' ''scalar (mathematics), scalars''. If v1,...,v''n'' are vectors and ''a''1,...,''a''''n'' are scalars, then the ''linear combination of those vectors with those scalars as coefficients'' is :a_1 \mathbf v_1 + a_2 \mathbf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
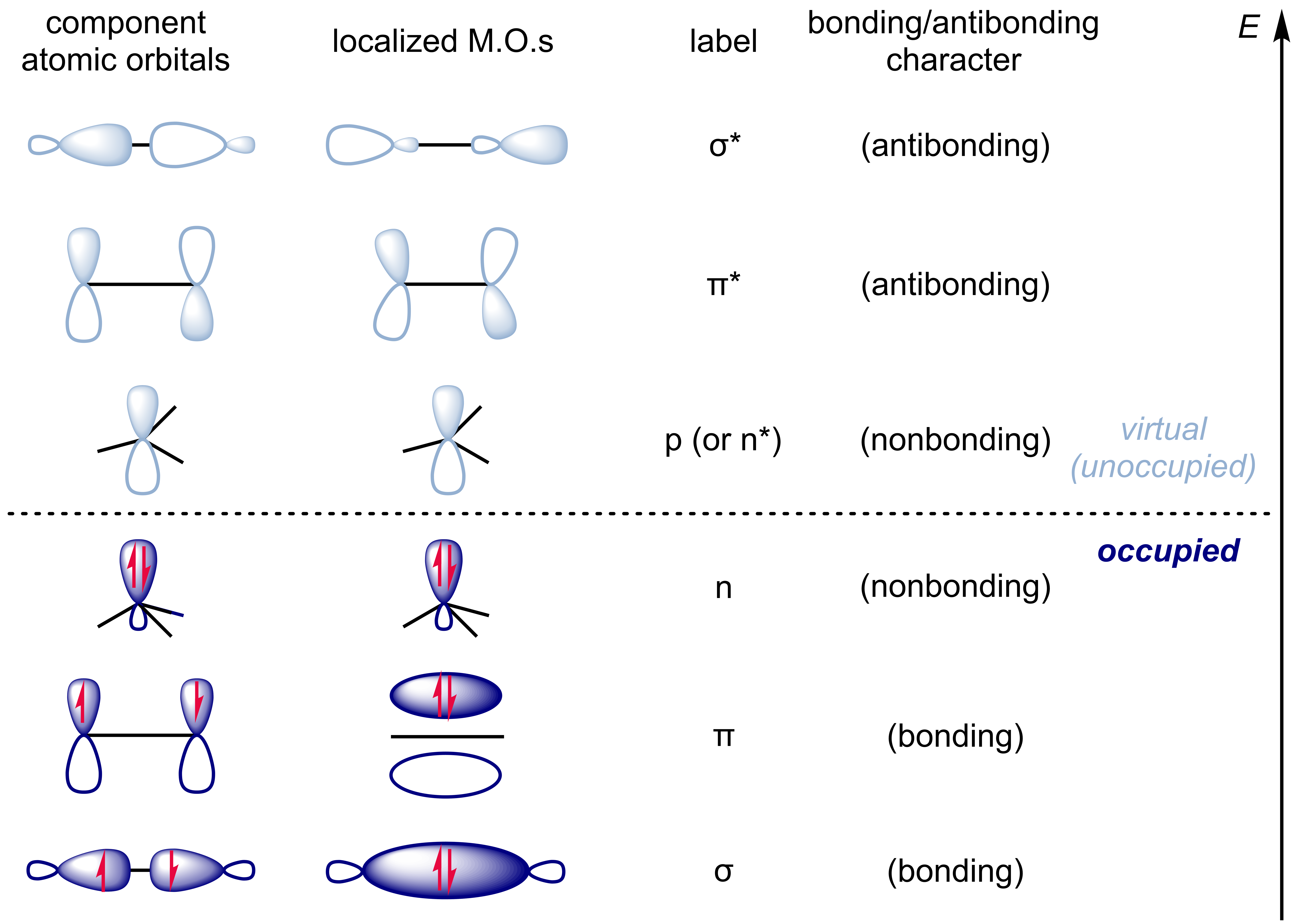
Localized Molecular Orbitals
Localized molecular orbitals are molecular orbitals which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation. Localized orbitals in systems with periodic boundary conditions are known as Wannier functions. Standard ab initio quantum chemistry methods lead to delocalized orbitals that, in general, extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combinations of the delocalized orbitals, given by an appropriate unitary transformation. In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The local ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ab Initio Quantum Chemistry Methods
''Ab initio'' quantum chemistry methods are a class of computational chemistry techniques based on quantum chemistry that aim to solve the electronic Schrödinger equation. ''Ab initio'' means "from first principles" or "from the beginning", meaning using only physical constants and the positions and number of electrons in the system as input. This ''ab initio'' approach contrasts with other computational methods that rely on empirical parameters or approximations. By solving this fundamental equation, ''ab initio'' methods seek to accurately predict various chemical properties, including electron densities, energies, and molecular structures. The ability to run these calculations has enabled theoretical chemists to solve a range of problems and their importance is highlighted by the awarding of the 1998 Nobel prize to John Pople and Walter Kohn. The term was first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Right Angle
In geometry and trigonometry, a right angle is an angle of exactly 90 Degree (angle), degrees or radians corresponding to a quarter turn (geometry), turn. If a Line (mathematics)#Ray, ray is placed so that its endpoint is on a line and the adjacent angles are equal, then they are right angles. The term is a calque of Latin ''angulus rectus''; here ''rectus'' means "upright", referring to the vertical perpendicular to a horizontal base line. Closely related and important geometrical concepts are perpendicular lines, meaning lines that form right angles at their point of intersection, and orthogonality, which is the property of forming right angles, usually applied to Euclidean vector, vectors. The presence of a right angle in a triangle is the defining factor for right triangles, making the right angle basic to trigonometry. Etymology The meaning of ''right'' in ''right angle'' possibly refers to the Classical Latin, Latin adjective ''rectus'' 'erect, straight, upright, perp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
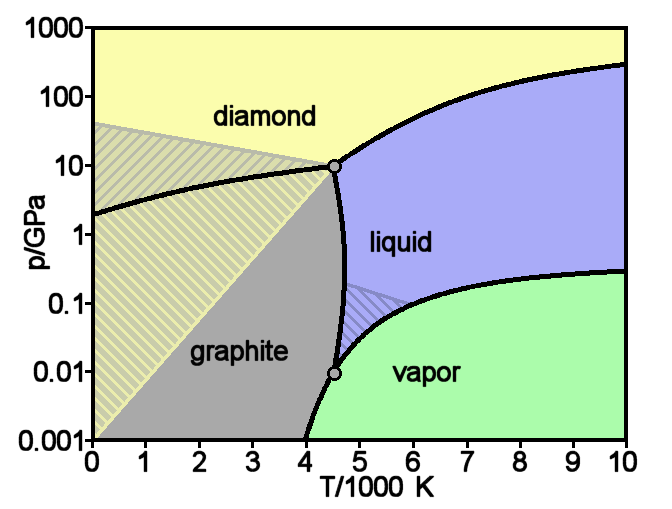
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of carbon at Standard temperature and pressure, room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest Scratch hardness, hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of lattice defect, defects or impurities (about one per million of lattice atoms) can color ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity indicates a material that readily allows electric current. Resistivity is commonly represented by the Greek alphabet, Greek letter (Rho (letter), rho). The SI unit of electrical resistivity is the ohm-metre (Ω⋅m). For example, if a solid cube of material has sheet contacts on two opposite faces, and the Electrical resistance, resistance between these contacts is , then the resistivity of the material is . Electrical conductivity (or specific conductance) is the reciprocal of electrical resistivity. It represents a material's ability to conduct electric current. It is commonly signified by the Greek letter (Sigma (letter), sigma), but (kappa) (especially in electrical engineering) and (gamma) are sometimes used. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |