|
Dehydroxylation
In chemistry, hydroxylation refers to the installation of a hydroxyl group () into an organic compound. Hydroxylations generate alcohols and phenols, which are very common functional groups. Hydroxylation confers some degree of water-solubility. Hydroxylation of a hydrocarbon is an oxidation, thus a step in degradation. Biological hydroxylation In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. These enzymes insert an O atom into a bond. Typical stoichiometries for the hydroxylation of a generic hydrocarbon are these: : : Since itself is a slow and unselective hydroxylating agent, catalysts are required to accelerate the pace of the process and to introduce selectivity. Hydroxylation is often the first step in the degradation of organic compounds in air. Hydroxylation is important in detoxification since it converts lipophilic compounds into water-soluble (hydrophilic) products that are more readily removed by the kidneys or liver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
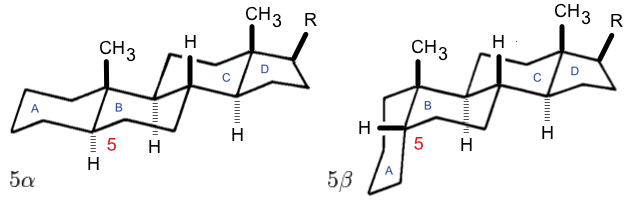
Steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signal transduction, signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in Fungus, fungi, plants, and animals. All steroids are manufactured in cells from a sterols, sterol: Cholesterol, cholesterol (animals), lanosterol (opisthokonts), or cycloartenol (plants). All three of these molecules are produced via Cyclic compound, cyclization of the triterpene squalene. Structure The steroid nucleus (parent structure, core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysyl 5-hydroxylase
Lysyl hydroxylases (or procollagen-lysine 5-dioxygenases) are alpha-ketoglutarate-dependent hydroxylases enzymes that catalyze the hydroxylation of lysine to hydroxylysine. Lysyl hydroxylases require iron and vitamin C as cofactors for their oxidation activity. It takes place (as a post-translational modification) following collagen synthesis in the cisternae (lumen) of the rough endoplasmic reticulum (ER). There are three lysyl hydroxylases (LH1-3) encoded in the human genome, namely: ''PLOD1'', ''PLOD2'' and ''PLOD3''. From ''PLOD2'' two splice variant can be expressed (LH2a and LH2b), where LH2b differs from LH2a by incorporating the small exon 13A. LH1 and LH3 hydroxylate lysyl residues in the collagen triple helix, whereas LH2b hydroxylates lysyl residues in the telopeptides of collagen. In addition to its hydroxylation activity, LH3 has glucosylation activity that produces disaccharide (Glc-Gal) attached to collagen hydroxylysines. Collagen lysyl hydroxylation is the firs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prolyl 3-hydroxylase
Procollagen-proline dioxygenase, commonly known as prolyl hydroxylase, is a member of the class of enzymes known as alpha-ketoglutarate-dependent hydroxylases. These enzymes catalyze the incorporation of oxygen into organic substrates through a mechanism that requires alpha-Ketoglutaric acid, Fe2+, and ascorbate. This particular enzyme catalyzes the formation of (2''S'', 4''R'')-4-hydroxyproline, a compound that represents the most prevalent post-translational modification in the human proteome. Enzyme mechanism Procollagen-proline dioxygenase catalyzes the following reaction: L-proline + alpha-ketoglutaric acid + O2 → (2''S'', 4''R'')-4-hydroxyproline + succinate + CO2 The mechanism for the reaction is similar to that of other dioxygenases, and occurs in two distinct stages: In the first, a highly reactive Fe(IV)=O species is produced. Molecular oxygen is bound end-on in an axial position, producing a dioxygen unit. Nucleophilic attack on C2 generates a tetrahedral in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypoxia Inducible Factor
Hypoxia-inducible factors (HIFs) are transcription factors that respond to decreases in available oxygen in the cellular environment, or hypoxia. They also respond to instances of pseudohypoxia, such as thiamine deficiency. Both hypoxia and pseudohypoxia leads to impairment of adenosine triphosphate (ATP) production by the mitochondria. Discovery The HIF transcriptional complex was discovered in 1995 by Gregg L. Semenza and postdoctoral fellow Guang Wang. In 2016, William Kaelin Jr., Peter J. Ratcliffe and Gregg L. Semenza were presented the Lasker Award for their work in elucidating the role of HIF-1 in oxygen sensing and its role in surviving low oxygen conditions. In 2019, the same three individuals were jointly awarded the Nobel Prize in Physiology or Medicine for their work in elucidating how HIF senses and adapts cellular response to oxygen availability. Structure Oxygen-breathing species express the highly conserved transcriptional complex HIF-1, which is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypoxia (medical)
Hypoxia is a condition in which the body or a region of the body is deprived of an adequate oxygen supply at the tissue (biology), tissue level. Hypoxia may be classified as either ''Generalized hypoxia, generalized'', affecting the whole body, or ''local'', affecting a region of the body. Although hypoxia is often a pathological condition, variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise. Hypoxia differs from hypoxemia and anoxemia, in that hypoxia refers to a state in which oxygen present in a tissue or the whole body is insufficient, whereas hypoxemia and anoxemia refer specifically to states that have low or no Oxygen saturation (medicine), oxygen in the blood. Hypoxia in which there is complete absence of oxygen supply is referred to as anoxia. Hypoxia can be due to external causes, when the breathing gas is hypoxic, or internal causes, such as reduced effectiveness of gas transfer in the lung ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
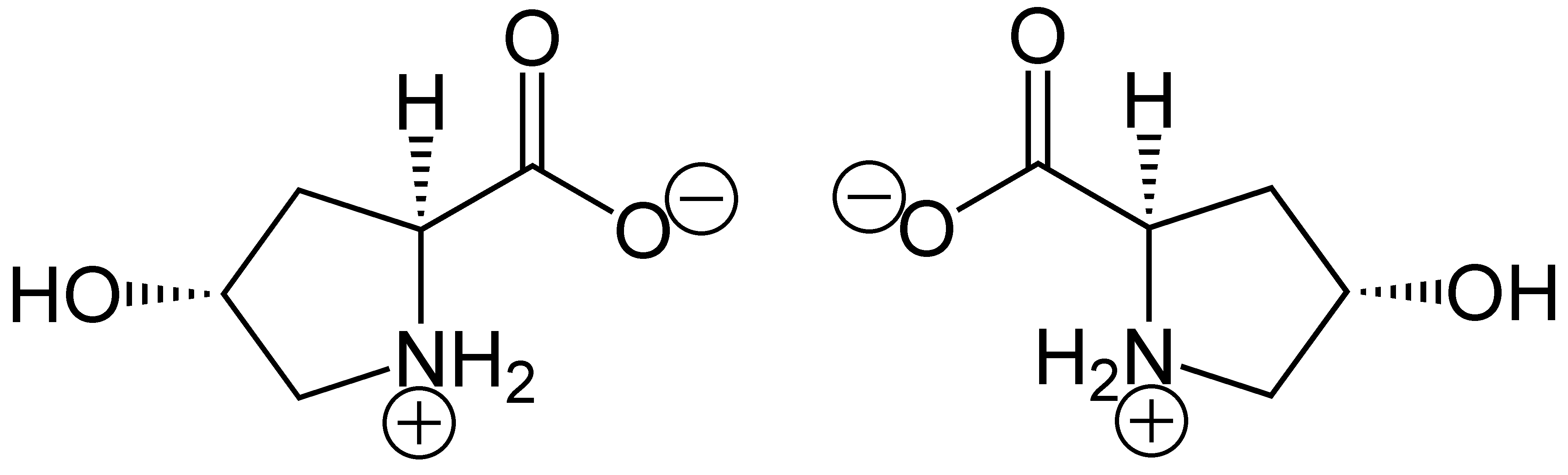
Hydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank. Structure and discovery In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelatin. In 1905, Hermann Leuchs synthesized a racemic mixture of 4-hydroxyproline. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom. Production and function Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl 4-hydroxylase following protein synthesis (as a post-translational modification). The enzyme-catalyzed reaction takes place in the lumen of the endoplasmic reticulum. Although it is not directly incorporated into proteins, hydroxyproline comprises roughly 4% of all amino acids found in animal tissue, an amount greater than seven other amino acids that are translationally incorporated. Animals Collagen Hydroxyproline is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis. Depending on the degree of biomineralization, mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the Gut (anatomy), gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes 1% to 2% of muscle tissue and 6% by weight of skeletal muscle. The fibroblast is the most common cell creating collagen in animals. Gelatin, which is used in food and industry, is collagen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translation (biology), translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signal transduction, signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C-terminus, C- or N-terminus, N- termini. They can expand the chemical set of the 22 proteinogenic amino acid, amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |