|
Dehalococcoides
''Dehalococcoides'' is a genus of bacteria within class Dehalococcoidia that obtain energy via the oxidation of hydrogen and subsequent reductive dehalogenation of halocarbon, halogenated organic compounds in a mode of anaerobic respiration called organohalide respiration. They are well known for their great potential to remediate halogenated ethenes and aromatics. They are the only bacteria known to transform highly chlorinated dioxins, PCBs. In addition, they are the only known bacteria to transform tetrachloroethene (Tetrachloroethylene, perchloroethene, PCE) to ethene. Microbiology The first member of the genus ''Dehalococcoides'' was described in 1997 as ''Dehalococcoides ethenogenes'' strain 195 (nom. inval.). Additional ''Dehalococcoides'' members were later described as strains CBDB1, BAV1, FL2, VS, and GT. In 2012 all yet-isolated ''Dehalococcoides'' strains were summarized under the new taxonomic name ''Dehalococcoides mccartyi, D. mccartyi'', with strain 195 as the type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halorespiration
Organohalide respiration (OHR) (previously named halorespiration or dehalorespiration) is the use of halogenated compounds as terminal electron acceptors in anaerobic respiration. Organohalide respiration can play a part in microbial biodegradation. The most common substrates are chlorinated aliphatics ( PCE, TCE, chloroform) and chlorinated phenols. Organohalide-respiring bacteria are highly diverse. This trait is found in some Campylobacterota, Thermodesulfobacteriota, Chloroflexota (green nonsulfur bacteria), low G+C gram positive Clostridia, and ultramicrobacteria. Process of organohalide respiration The process of organohalide respiration, uses reductive dehalogenation to produce energy that can be used by the respiring microorganism to carry out its growth and metabolism. Halogenated organic compounds are used as the terminal electron acceptor, which results in their dehalogenation. Reductive dehalogenation is the process by which this occurs. It involves the reducti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductive Dehalogenation
In organochlorine chemistry, reductive dechlorination describes any chemical reaction which cleaves the covalent bond between carbon and chlorine via reductants, to release chloride ions. Many modalities have been implemented, depending on the application. Reductive dechlorination is often applied to remediation of chlorinated pesticides or dry cleaning solvents. It is also used occasionally in the synthesis of organic compounds, e.g. as pharmaceuticals. Chemical Dechlorination is a well-researched reaction in organic synthesis, although it is not often used. Usually stoichiometric amounts of dechlorinating agent are required. In one classic application, the Ullmann reaction, chloroarenes are coupled to biphenyls. For example, the activated substrate 2-chloronitrobenzene is converted into 2,2'-dinitrobiphenyl with a copper - bronze alloy. Zerovalent iron effects similar reactions. Organophosphorus(III) compounds effect gentle dechlorinations. The products are alkenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloroethylene
Trichloroethylene (TCE) is an organochloride with the formula C2HCl3, commonly used as an industrial metal-degreasing solvent. It is a clear, colourless, non-flammable, volatile liquid with a chloroform-like pleasant mild smell and sweet taste.Trichloroethylene (TCE) on ATSDR Its name is trichloroethene. Trichloroethylene has been sold under a variety of trade names. Industrial abbreviations include trichlor, Trike, Tricky and tri. Under the trade names Trimar and Trilene, it was used as a [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloroethylene
Trichloroethylene (TCE) is an organochloride with the formula C2HCl3, commonly used as an industrial metal-degreasing solvent. It is a clear, colourless, non-flammable, volatile liquid with a chloroform-like pleasant mild smell and sweet taste.Trichloroethylene (TCE) on ATSDR Its name is trichloroethene. Trichloroethylene has been sold under a variety of trade names. Industrial abbreviations include trichlor, Trike, Tricky and tri. Under the trade names Trimar and Trilene, it was used as a [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
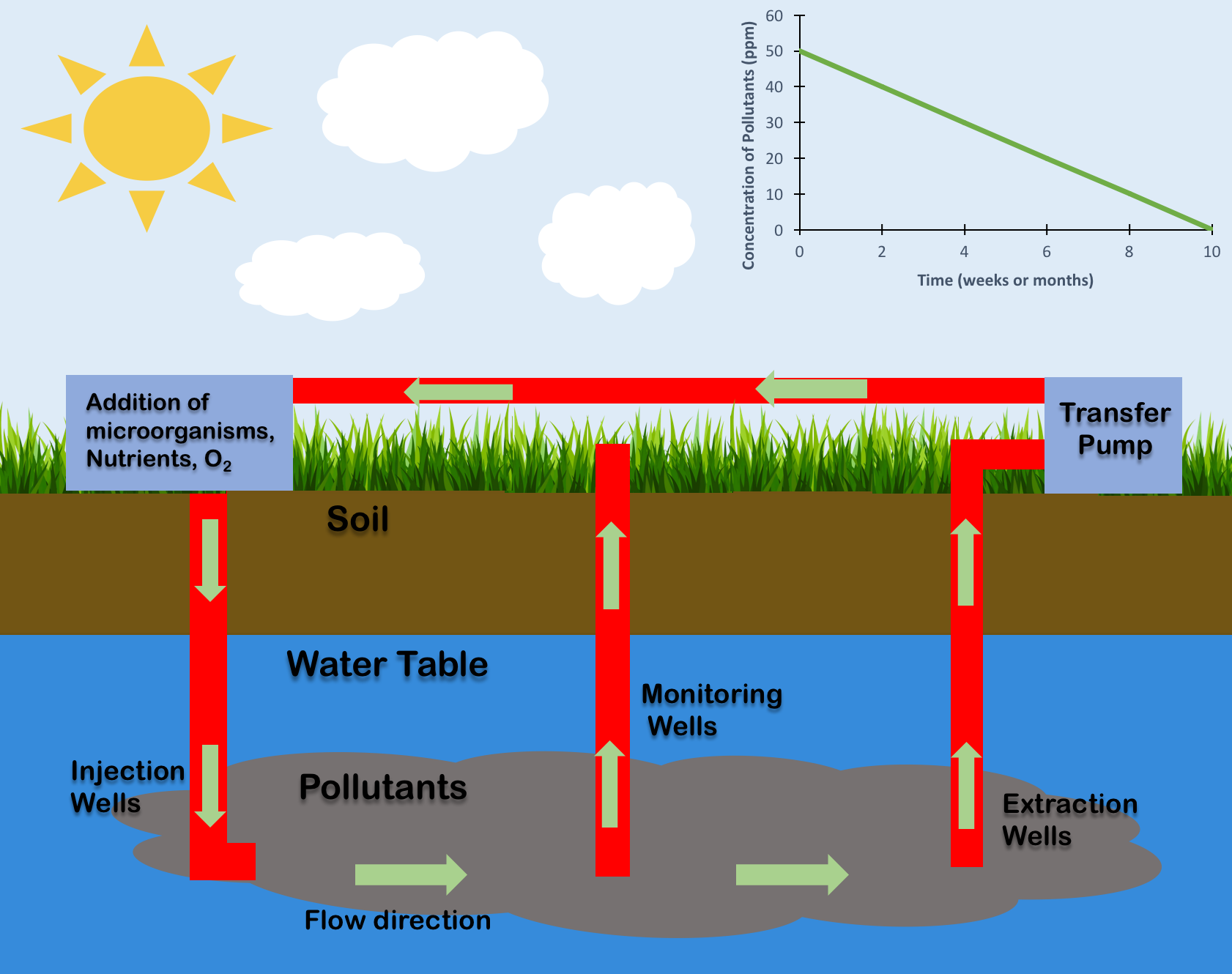
Bioremediation
Bioremediation broadly refers to any process wherein a biological system (typically bacteria, microalgae, fungi in mycoremediation, and plants in phytoremediation), living or dead, is employed for removing environmental pollutants from air, water, soil, fuel gasses, industrial effluents etc., in natural or artificial settings. The natural ability of organisms to adsorb, accumulate, and degrade common and emerging pollutants has attracted the use of biological resources in treatment of contaminated environment. In comparison to conventional physicochemical treatment methods bioremediation may offer advantages as it aims to be sustainable, eco-friendly, cheap, and scalable. This technology is rarely implemented however because it is slow or inefficient. Most bioremediation is inadvertent, involving native organisms. Research on bioremediation is heavily focused on stimulating the process by inoculation of a polluted site with organisms or supplying nutrients to promote their growt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anaerobic Respiration
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain. In aerobic organisms undergoing respiration, electrons are shuttled to an electron transport chain, and the final electron acceptor is oxygen. Molecular oxygen is an excellent electron acceptor. Anaerobes instead use less-oxidizing substances such as nitrate (), fumarate (), sulfate (), or elemental sulfur (S). These terminal electron acceptors have smaller reduction potentials than O2. Less energy per oxidized molecule is released. Therefore, anaerobic respiration is less efficient than aerobic. As compared with fermentation Anaerobic cellular respiration and fermentation generate ATP in very different ways, and the terms should not be treated as synonyms. Cellular respiration (both aerobic and anaerobic) uses highly reduced chemical compounds such as NADH and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polychlorinated Biphenyl
Polychlorinated biphenyls (PCBs) are organochlorine compounds with the formula Carbon, C12Hydrogen, H10−''x''Chloride, Cl''x''; they were once widely used in the manufacture of carbonless copy paper, as heat transfer fluids, and as dielectric and coolant fluids for electrical equipment. They are highly toxic and carcinogenic chemical compounds, formerly used in industrial and consumer electronic products, whose production was banned internationally by the Stockholm Convention on Persistent Organic Pollutants in 2001. Because of their longevity, PCBs are still widely in use, even though their manufacture has declined drastically since the 1960s, when a multitude of problems was identified. With the discovery of PCBs' environmental toxicity, and classification as persistent organic pollutants, their production was banned for most uses by United States federal law on January 1, 1978. The International Agency for Research on Cancer (IARC) rendered PCBs as definite carcinogens i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |