|
DIOP
DIOP (2,3-''O''-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino)butane) is an organophosphorus compound that is used as a chiral ligand in asymmetric catalysis. It is a white solid that is soluble in organic solvents. DIOP is prepared from the acetonide of ''d,l''-tartaric acid, which is reduced prior to attachment of the PPh2 substituents. Use The DIOP ligand binds to metals via conformationally flexible seven-membered C4P2M chelate ring. DIOP is a historically important in the development of ligands for use in asymmetric catalysis, an atom-economical method for the preparation of chiral compounds. Described in 1971, it was the first example of a C2-symmetric diphosphine. Its complexes have been applied to the reduction of prochiral olefins, ketones, and imines. Knowles et al. independently reported the related C2-symmetric diphosphine DIPAMP. Since the discovery of DIOP, many analogues of DIOP have been introduced. These DIOP derivatives include MOD-DIOP, Cy- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligand, phosphine ligands in inorganic chemistry, inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelate, chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in Homogeneous catalysis, homogeneous catalysts. Synthesis image:IPr2PCl.png, 220px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
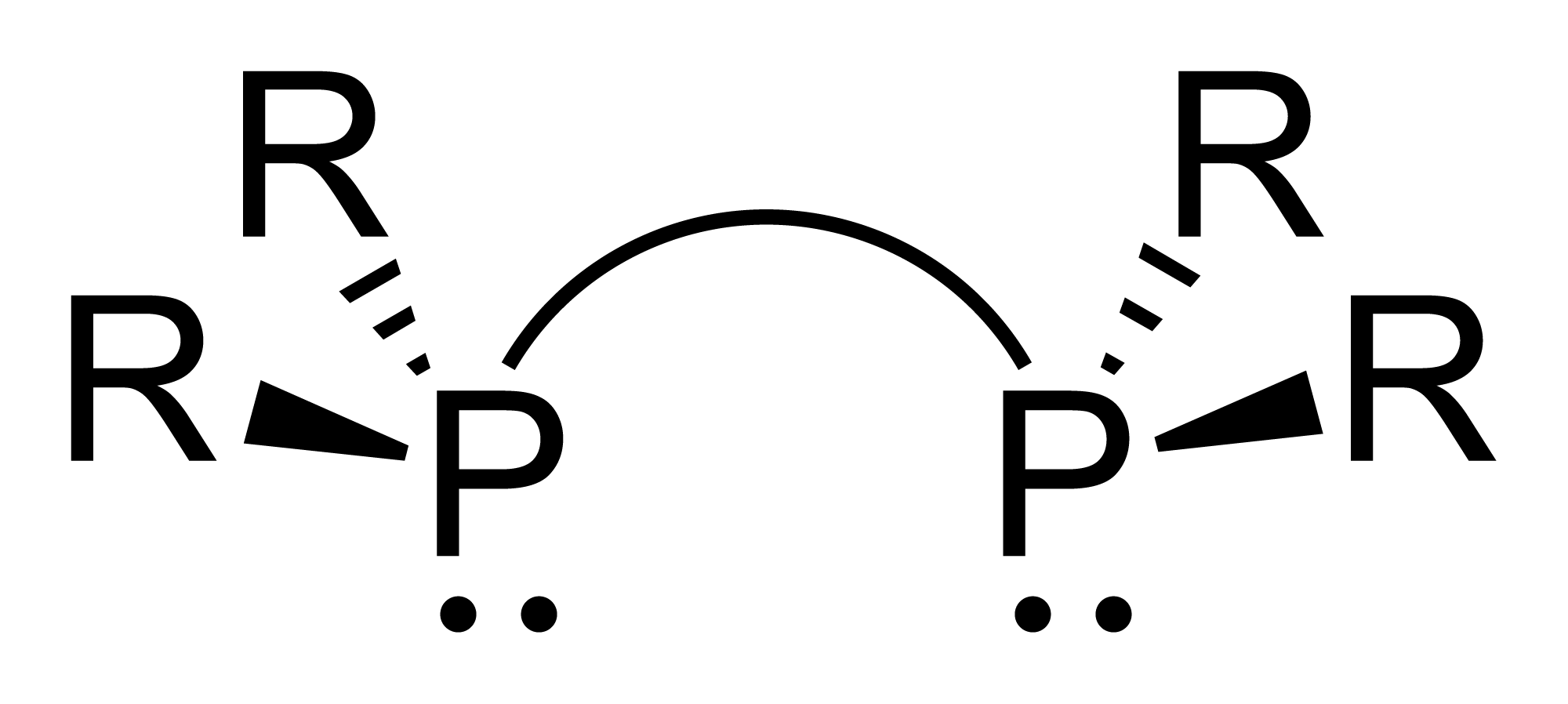
C2-Symmetric Ligands
In homogeneous catalysis, ''C''2-symmetric ligands refer to ligands that lack mirror symmetry but have ''C''2 symmetry (two-fold rotational symmetry). Such ligands are usually bidentate and are valuable in catalysis. The ''C''2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. ''C''2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including ''C''2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product. Examples An early ''C''2-symmetric ligand, diphosphine catalytic ligand DIPAMP, was developed in 1968 by William S. Knowles and coworkers of Monsanto Company, who shared the 2001 Nobel Prize in Chemistry. This ligand was used in the industrial production of -DOPA. : Some classes of ''C''2-symmetric li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Distinction is sometimes made between aldimines and ketimines, derived from aldehydes and ketones, respectively. Structure In imines the five core atoms (C2C=NX, ketimine; and C(H)C=NX, aldimine; X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29–1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C−N distances in amines and nitriles are 1.47 and 1.16 Å respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E–Z notation, ''E'' and ''Z'' isomers of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonide
In organic chemistry, an acetonide is the functional group composed of the cyclic ketal of a diol with acetone. The more systematic name for this structure is an isopropylidene ketal. Acetonide is a common protecting group for 1,2- and 1,3-diols. The protecting group can be removed by hydrolysis of the ketal using dilute aqueous acid. Example The acetonides of small di- and triols, as well as many sugars and sugar alcohols, are common. The hexaol mannitol reacts with 2,2-dimethoxypropane to give the bis-acetonide, which oxidizes to give the acetonide of glyceraldehyde: :(CHOHCHOHCH2OH)2 + 2 (MeO)2CMe2 → (CHOHCHCH2O2CMe2)2 + 4 MeOH :(CHOHCHOCH2OCMe2)2 + → 2 OCHCHCH2O2CMe2 + H2O An example of its use as a protecting group in a complex organic synthesis is the Nicolaou Taxol total synthesis. It is a common protecting group for sugars and sugar alcohols, a simple example being solketal. The acetonides of corticosteroid are used in dermatology, because their increased lipop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tartaric Acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus. Its salt (chemistry), salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of winemaking, fermentation. Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E-numbers, E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic acid, diprotic and aldaric acid, aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid. History Tartaric acid has been known to winemakers for centuries. However, the chemical process for extraction was developed in 1769 by the Sweden, Swedish chemist Carl Wilhel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelate Ring
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity. The word ''chelation'' is derived from Greek χηλή, ''chēlē'', meaning "claw"; the ligands lie around the central atom like the claws of a crab. The term ''chelate'' () was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or ''chele'' (Greek) of the crab or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings." Chelation is useful in applications such as providing nutritional supplements, in chela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom Economy
Atom economy (atom efficiency/percentage) is the conversion efficiency of a chemical process in terms of all atoms involved and the desired products produced. The simplest definition was introduced by Barry Trost in 1991 and is equal to the ratio between the mass of desired product to the total mass of reactants, expressed as a percentage. The concept of atom economy (AE) and the idea of making it a primary criterion for improvement in chemistry, is a part of the green chemistry movement that was championed by Paul Anastas from the early 1990s. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the "greenness" of a process or synthesis. Good atom economy means most of the atoms of the reactants are incorporated in the desired products and only small amounts of unwanted byproducts are formed, reducing the economic and environmental impact of waste disposal. Atom economy can be written as: \text = \frac \tim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Catalysis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of Chirality (chemistry), chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereomeric) products in Enantiomeric excess, unequal amounts." Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers. Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity. Overview Many of the building blocks of biological systems such as sugars a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William Standish Knowles
William Standish Knowles (June 1, 1917 – June 13, 2012) was an American chemist. He was born in Taunton, Massachusetts. Knowles was one of the recipients of the 2001 Nobel Prize in Chemistry. He split half the prize with Ryōji Noyori for their work in asymmetric synthesis, specifically for his work in hydrogenation reactions. The other half was awarded to K. Barry Sharpless for his work in oxidation reactions. Education Knowles attended Berkshire School in Sheffield, Massachusetts. He led his class academically and upon graduation was admitted to Harvard University. Feeling that he was too young to go to college, Knowles spent a year at Phillips Academy in Andover, Massachusetts. At the end of the year, he captured his first award in chemistry, the school's $50 Boylston Prize. After his year in preparatory school, Knowles attended Harvard, where he majored in chemistry, focusing on organic chemistry. He received his undergraduate degree in 1939, and attended Columbi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DIPAMP
DIPAMP is an organophosphorus compound that is used as a ligand in homogeneous catalysis. It is a white solid that dissolves in organic solvents. Work on this compound by W. S. Knowles was recognized with the Nobel Prize in Chemistry. DIPAMP was the basis for one of the first industrial scale asymmetric hydrogenation, the synthesis of the drug L-DOPA. : DIPAMP is a C2-symmetric diphosphine. Each phosphorus Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ... centre, which is pyramidal, bears three different substituents - anisyl, phenyl, and the ethylene group. The ligand therefore exists as the enantiomeric (''R'',''R'') and (''S'',''S'') pair, as well as the achiral ''meso'' isomer. DIPAMP was originally prepared by an oxidative coupling, starting from anisyl(phenyl)(methyl)p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantioselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite: both products are at least possible and merely differ in amount. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used. An enantioselective reaction is one in which one enantiomer is formed in preference to the other, in a reaction that creates an optically active product from an achiral starting material, using either a chiral catalyst, an enzyme or a chiral reagent. The degree of selectivity is m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |