|
DIDS
4,4′-Diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) is an anion exchange inhibitor Inhibitor or inhibition may refer to: In biology * Enzyme inhibitor, a substance that binds to an enzyme and decreases the enzyme's activity * Reuptake inhibitor, a substance that increases neurotransmission by blocking the reuptake of a neurotra ..., blocking reversibly, and later irreversibly, exchangers such as chloride-bicarbonate exchanger. References Sulfonic acids Isothiocyanates Alkene derivatives {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion Exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances. Ion exchange usually describes a process of purification of aqueous solutions using solid polymeric ion-exchange resin. More precisely, the term encompasses a large variety of processes where ions are exchanged between two electrolytes. Aside from its use to purify drinking water, the technique is widely applied for purification and separation of a variety of industrially and medicinally important chemicals. Although the term usually refers to applications of synthetic (man-made) resins, it can include many other materials such as soil. Typical ion exchangers are ion-exchange resins (functionalized porous or gel polymer), zeolites, montmorillonite, clay, and soil humus. Ion ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Inhibitor
A reaction inhibitor is a substance that decreases the rate of, or prevents, a chemical reaction. A catalyst, in contrast, is a substance that increases the rate of a chemical reaction. Examples * Added acetanilide slows the decomposition of drug-store hydrogen peroxide solution, inhibiting the reaction 2 → 2 + , which is catalyzed by heat, light, and impurities.The decomposition of hydrogen peroxide Inhibition of a catalyst An inhibitor can reduce the effectiveness of a catalyst in a catalysed reaction (either a non-biological catalyst or an enzyme). E.g., if a compound is so similar to (one of) the reactants that it can bind to the active site of a catalyst but does not undergo a catalytic reaction then that catalyst molecule cannot perform its job because the active site is occupied. When the inhibitor is released, the catalyst is again available for reaction. Inhibition and catalyst poisoning Inhibition should be distinguished from catalyst poisoning. An inhibitor only hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Membrane Biology
''The Journal of Membrane Biology'' is a monthly peer-reviewed scientific journal on the nature, structure, genesis, and functions of biological membranes and on the physics and chemistry Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ... of artificial membranes with a bearing on biomembranes. According to the '' Journal Citation Reports'', the journal has a 2022 impact factor of 2.4. References External links * {{DEFAULTSORT:Journal Of Membrane Biology, The English-language journals Biology journals Springer Science+Business Media academic journals Monthly journals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
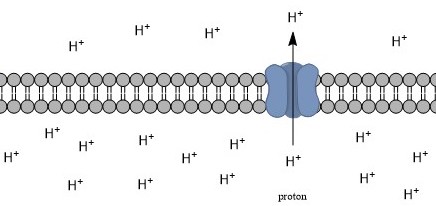
Exchanger (protein)
An antiporter (also called exchanger or counter-transporter) is a cotransporter and integral membrane protein involved in secondary active transport of two or more different molecules or ions across a phospholipid membrane such as the plasma membrane in opposite directions, one into the cell and one out of the cell. Na+/H+ antiporters have been reviewed. In secondary active transport, one species of solute moves along its electrochemical gradient, allowing a different species to move against its own electrochemical gradient. This movement is in contrast to primary active transport, in which all solutes are moved against their concentration gradients, fueled by ATP. Transport may involve one or more of each type of solute. For example, the Na+/Ca2+ exchanger, found in the plasma membrane of many cells, moves three sodium ions in one direction, and one calcium ion in the other. Role in Homeostatic Mechanisms Na+/H+ Antiporters Antiporters, such as Na+/H+ antiporter protein, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intracellular PH
Intracellular pH (pHi) is the measure of the acidity or basicity (i.e., pH) of intracellular fluid. The pHi plays a critical role in membrane transport and other intracellular processes. In an environment with the improper pHi, biological cells may have compromised function. Therefore, pHi is closely regulated in order to ensure proper cellular function, controlled cell growth, and normal cellular processes. The mechanisms that regulate pHi are usually considered to be plasma membrane transporters of which two main types exist — those that are dependent and those that are independent of the concentration of bicarbonate (). Physiologically normal intracellular pH is most commonly between 7.0 and 7.4, though there is variability between tissues (e.g., mammalian skeletal muscle tends to have a pHi of 6.8–7.1). There is also pH variation across different organelles, which can span from around 4.5 to 8.0. pHi can be measured in a number of different ways. Homeostasis Intracellul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biology Of Reproduction
''Biology of Reproduction'' is a peer-reviewed scientific journal and the official journal of the Society for the Study of Reproduction. It is published with the assistance of Oxford University Press. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as ... of 4.285, ranking it 5th out of 29 journals in the category "Reproductive Biology". References External links * Developmental biology journals English-language journals Publications established in 1969 Monthly journals Academic journals published by learned and professional societies {{biology-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acids
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkylsul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isothiocyanates
In organic chemistry, isothiocyanate is the functional group , formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also known as mustard oils. An artificial isothiocyanate, phenyl isothiocyanate, is used for amino acid sequencing in the Edman degradation. Cruciferous vegetables, such as bok choy, broccoli, cabbage, cauliflower, kale, and others, are rich sources of glucosinolate precursors of isothiocyanates. Although there has been some basic research on how isothiocyanates might exert biological effects ''in vivo'', there is no high-quality evidence to date for its efficacy against human diseases. Structure Typical bond angles for and linkages in aryl isothiocyanates are 165° and 177°, respectively. The and distances are 117 and 158 pms. Synthesis and reactions Isot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |