|
Trifluoromethyl Ethers
The trifluoromethyl group is a functional group that has the formula . The naming of is group is derived from the methyl group (which has the formula ), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane , 1,1,1-trifluoroethane , and hexafluoroacetone . Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from 1928, although res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Medicinal And Pharmaceutical Chemistry
The ''Journal of Medicinal Chemistry'' is a biweekly peer-reviewed medical journal covering research in medicinal chemistry. It is published by the American Chemical Society. It was established in 1959 as the ''Journal of Medicinal and Pharmaceutical Chemistry'' and obtained its current name in 1963. Philip S. Portoghese served as editor-in-chief from 1972 to 2011. In 2012, Gunda Georg (University of Minnesota) and Shaomeng Wang (University of Michigan) succeeded Portoghese (University of Minnesota). In 2021, Craig W. Lindsley (Vanderbilt University) became editor-in-chief. According to the ''Journal Citation Reports'', the journal has a 2023 impact factor of 7.1. See also *ACS Medicinal Chemistry Letters ''ACS Medicinal Chemistry Letters'' is a monthly peer-reviewed scientific journal covering medicinal chemistry. It was established in 2009 and is published by the American Chemical Society. The editor-in-chief is Dennis C. Liotta (Emory University ... References External l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Trifluoride
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, it is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry. Preparation and structure In solid SbF3, the Sb centres have octahedral molecular geometry and are linked by bridging fluoride ligands. Three Sb–F bonds are short (192 pm) and three are long (261 pm). Because it is a polymer, SbF3 is far less volatile than related compounds AsF3 and SbCl3. SbF3 is prepared by treating antimony trioxide with hydrogen fluoride: :Sb2O3 + 6 HF → 2 SbF3 + 3 H2O The compound is a mild Lewis acid, hydrolyzing slowly in water. With fluorine, it is oxidized to give antimony pentafluoride. :SbF3 + F2 → SbF5 Applications It is used as a fluorination reagent in organic chemistry. This application was reported by the Belgian c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
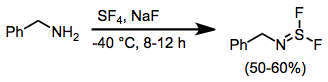
Sulfur Tetrafluoride
Sulfur tetrafluoride is a chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the +4 oxidation state, with one lone pair of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g., alkyl, alkenyl, aryl), or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They often have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid () is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenoxy Herbicide
Phenoxy herbicides (or "phenoxies") are two families of chemicals that have been developed as commercially important herbicides, widely used in agriculture. They share the part structure of phenoxyacetic acid. Auxins The first group to be discovered act by mimicking the auxin growth hormone indoleacetic acid (IAA). When sprayed on broad-leaf plants they induce rapid, uncontrolled growth ("growing to death"). Thus when applied to monocotyledonous crops such as wheat or maize (corn), they selectively kill broad-leaf weeds, leaving the crops relatively unaffected. File:Indol-3-ylacetic acid.svg, IAA File:2-(4-chloro-2-methylphenoxy)acetic acid 200.svg, MCPA File:2-(2,4-dichlorophenoxy)acetic acid 200.svg, 2,4-D File:2-(2,4,5-trichlorophenoxy)acetic acid 200.svg, 2,4,5-T Introduced in 1946, these herbicides were in widespread use in agriculture by the middle of the 1950s. The best known phenoxy herbicides are (4-chloro-2-methylphenoxy)acetic acid (MCPA), 2,4-dichlorophenoxyacetic aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluazifop
Fluazifop is the trivial name, common name used by the International Organization for Standardization, ISO for an organic compound that is used as a selective herbicide. The active ingredient is the 2R enantiomer at its chiral centre and this material is known as fluazifop-P when used in that form. More commonly, it is sold as its butyl ester, fluazifop-P butyl with the brand name Fusilade. History In the 1970s, a number of agrochemical companies were working to develop new herbicides to be complementary to the auxin phenoxyacetic acid types such as 2,4-D, which had activity on broad-leaved weeds but were safe to grass crops such as the cereals. Thus the aim was to find materials which would selectively control grass weeds in broad-leaved crops such as cotton and soybean. In 1973, Hoechst AG filed patents on a new class of compound, the aryloxyphenoxypropionates, which showed such selectivity and led to the commercialisation of diclofop. Then the Japanese company Ishihara Sangyo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluralin
Trifluralin is a common pre-emergent selective herbicide, a dinitroaniline. With about used in the United States in 2001, and in 2012, it is one of the most widely used herbicides. Trifluralin is also used in Australia, New Zealand, Brazil and previously in the EU. Introduced in 1964, Trifluralin was the first organofluorine compound used as an agrochemical. Trifluralin is generally applied to the soil to control annual grass and broadleaf weed species. It inhibits root development by interrupting mitosis and controls weeds as they germinate. Trifluralin moves very little inside the plant, remaining in the roots. Discovery Selective herbicides were unavailable in the 1950s to protect soybean and cotton ( 2,4-DNP could have been used but had to be exactingly applied lest it destroy the crops), so Lilly Research Laboratories screened ~2000 compounds from 1958 to 1980 blindly looking for a result. Trifluralin was initially thought a failure, yet the plots stayed free of w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoxaflor
Sulfoxaflor, also marketed as ''Isoclast'', is a systemic insecticide that acts as an insect neurotoxin. A pyridine and a trifluoromethyl compound, it is a member of a class of chemicals called sulfoximines, which act on the central nervous system of insects. Mechanism of action Sulfoxaflor is a systemic insecticide, acts as a neurotoxin to affected insects, and kills through contact or ingestion. Sulfoxaflor is classified for use against sap-feeding insects as a sulfoximine, which is a sub-group of insecticides that act as nicotinic acetylcholine receptor (nAChR) competitive modulators. Sulfoxaflor binds to nAChRs in place of acetylcholine. Sulfoxaflor binding causes uncontrolled nerve impulses resulting in muscle tremors followed by paralysis and death. Other nAChR competitive modulator sub-groups that bind differently on the receptor than sulfoximines include neonicotinoids, nicotine, and butenolides. Because sulfoxaflor binds much more strongly to insect neuron receptors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
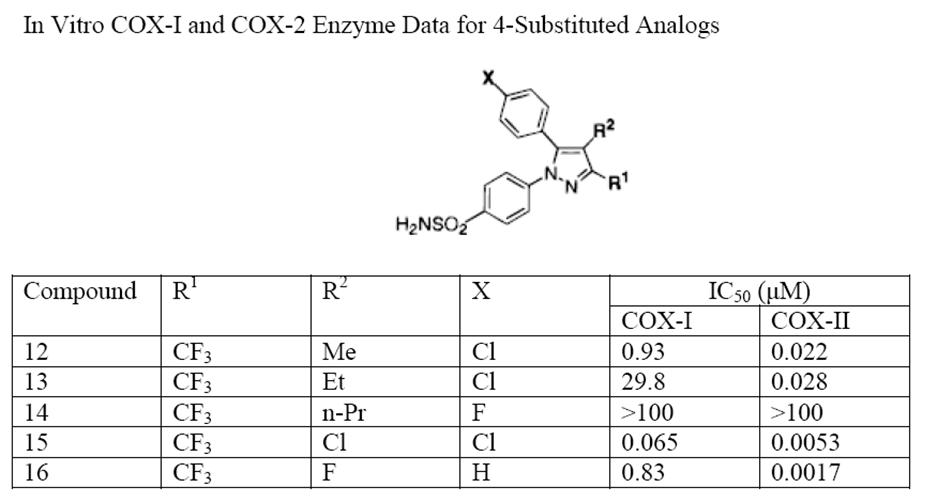
Nonsteroidal Anti-inflammatory Drug
Non-steroidal anti-inflammatory drugs (NSAID) are members of a Indication (medicine), therapeutic drug class which Analgesic, reduces pain, Anti-inflammatory, decreases inflammation, Antipyretic, decreases fever, and Antithrombotic, prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of Stomach ulcers, gastrointestinal ulcers and bleeds, heart attack, and kidney disease. The term ''non-steroidal'', common from around 1960, distinguishes these drugs from corticosteroids, another class of anti-inflammatory drugs, which during the 1950s had acquired a bad reputation due to overuse and side-effect problems after their introduction in 1948. NSAIDs work by inhibiting the activity of cyclooxygenase enzymes (the COX-1 and COX-2 isozyme, isoenzymes). In cells, these enzymes are involved in the synthesis of key biological mediators, namely prostaglandins, which are involved in inflammation, and thromboxanes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celecoxib
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID). It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis. It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis. It is taken by mouth. Benefits are typically seen within an hour. Common side effects include abdominal pain, nausea, and diarrhea. Serious side effects may include heart attacks, strokes, gastrointestinal perforation, gastrointestinal bleeding, kidney failure, and anaphylaxis. Use is not recommended in people at high risk for heart disease. The risks are similar to other NSAIDs, such as ibuprofen and naproxen. Use in the later part of pregnancy or during breastfeeding is not recommended. Celecoxib has demonstrated adjunctive bene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoxetine
Fluoxetine, sold under the brand name Prozac, among others, is an Antidepressant, antidepressant medication of the selective serotonin reuptake inhibitor (SSRI) class used for the treatment of major depressive disorder, Anxiety disorder, anxiety, obsessive–compulsive disorder (OCD), panic disorder, premenstrual dysphoric disorder, and bulimia nervosa. It is also approved for treatment of major depressive disorder in adolescents and children 8 years of age and over. It has also been used to treat premature ejaculation. Fluoxetine is oral administration, taken by mouth. Common side effects include anorexia (symptom), loss of appetite, nausea, diarrhea, headache, insomnia, trouble sleeping, xerostomia, dry mouth, and sexual dysfunction. Serious side effects include serotonin syndrome, mania, seizures, an increased risk of suicide, suicidal behavior in people under 25 years old, and an increased risk of bleeding. Antidepressant discontinuation syndrome is less likely to occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |