|
Tetrahydroisoquinolines
A substituted tetrahydroisoquinoline is a tetrahydroisoquinoline with one or more chemical substituents. Many simple tetrahydroisoquinoline alkaloids related to mescaline are known and occur naturally in cactus species such as peyote (''Lophophora williamsii'') and ''Pachycereus pringlei'' among many others. Simple tetrahydroisoquinolines may be thought of as cyclized phenethylamines. As an example, anhalinine may be thought of as a cyclized analogue of mescaline. The simple tetrahydroisoquinolines are analogous in concept to the β-carbolines and harmala alkaloids, which can be considered cyclized analogues of tryptamines. Some of the simple tetrahydroisoquinolines, for instance pellotine, are known to be pharmacologically active, although none are known to have hallucinogenic activity. Known activities of simple tetrahydroisoquinolines include sedative and hypnotic effects, monoamine oxidase inhibition, and convulsant effects, among various others. In the 2020s, various s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydroisoquinoline
Tetrahydroisoquinoline (TIQ or THIQ), also known as AMPH-CR, is an organic compound with the chemical formula C9H11N. Classified as a secondary amine, it is derived from isoquinoline by hydrogenation. It is a colorless viscous liquid that is miscible with most organic solvents. The tetrahydroisoquinoline skeleton is encountered in a number of bioactive compounds and drugs. Pharmacology THIQ is a conformationally restrained (CR) or cyclized analogue of β-phenethylamine and amphetamine and is also known as AMPH-CR. In contrast to amphetamine however, THIQ fails to substitute for dextroamphetamine in rodent drug discrimination tests, suggesting that it lacks stimulant effects. Similar findings have been made for other tetrahydroisoquinoline analogues of psychoactive phenethylamines, for instance DOM-CR. In any case, THIQ does substitute for TDIQ (MDTIQ), a selective α2-adrenergic receptor ligand, indicating that it is not pharmacologically inactive. Reactions As a secon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT7 Receptor
The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the ''HTR7'' gene, which in humans is transcribed into 3 different splice variants. Function When the 5-HT7 receptor is activated by serotonin, it sets off a cascade of events starting with release of the stimulatory G protein Gs from the GPCR complex. Gs in turn activates adenylate cyclase which increases intracellular levels of the second messenger cAMP. The 5-HT7 receptor plays a role in smooth muscle relaxation within the vasculature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhalinine
Anhalinine, also known as ''O''-methylanhalamine or mescaline-CR, is a tetrahydroisoquinoline alkaloid found in ''Lophophora williamsii'' (peyote) and other cacti. It is structurally related to mescaline and is a cyclized phenethylamine analogue of mescaline. Anhalinine is also pharmacologically active, but is only a minor constituent of peyote and is unlikely to contribute to its effects. Simple isoquinoline alkaloids of mescaline-containing cacti like anhalinine have received relatively little investigation. Arthur Heffter found many of them to produce no effects similar to those of mescaline. However, some of them have been found to produce convulsions in animals at high doses. Anhalinine specifically has been described as having "stimulant" properties due to inhibiting cholinergic neurotransmission. Alexander Shulgin tried anhalinine at small doses of 0.5 to 4.3mg but experienced no effects.Alexander Shulgin. Pharmacology Notebook 1. Subacute effects Anhalinine. 1963. https ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pellotine
Pellotine, also known as peyotline or ''N''-methylanhalonidine, is a tetrahydroisoquinoline alkaloid found in '' Lophophora'' species, in particular ''L. diffusa''. It is the second most common alkaloid found in ''Lophophora williamsii'' (peyote). Pellotine is slightly sedative, and has been used by Native Americans as a constituent of peyote for sacramental purposes. It was reportedly once marketed for use as a sedative. Pharmacology and effects Doses of 8 to 10mg of isolated pellotine are known to cause convulsions in frogs. When injected subcutaneously to humans, participants have reported drowsiness and a desire not to exert any physical or mental effort, with one study reporting it to have hypnotic effects. It is also reported to lower blood pressure and heart rate. Pellotine produced no hallucinogenic effects in humans at doses of up to 250mg. However, it has been reported to have a calming or sedative effect instead. Pellotine has been identified as a selective an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Agonist
In pharmacology, partial agonists are drugs that bind to and activate a given Receptor (biochemistry), receptor, but have only partial Intrinsic activity, efficacy at the receptor relative to a full agonist. They may also be considered Ligand (biochemistry), ligands which display both agonistic and Receptor antagonist, antagonistic effects—when both a full agonist and partial agonist are present, the partial agonist actually acts as a competitive antagonist, competing with the full agonist for receptor occupancy and producing a net decrease in the receptor activation observed with the full agonist alone. Clinically, partial agonists can be used to activate receptors to give a desired submaximal response when inadequate amounts of the endogenous ligand are present, or they can reduce the overstimulation of receptors when excess amounts of the endogenous ligand are present. Some currently common drugs that have been classed as partial agonists at particular receptors include buspi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT6 Receptor
The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. ''HTR6'' denotes the human gene encoding for the receptor. Distribution The 5HT6 receptor is expressed almost exclusively in the brain. It is distributed in various areas including, but not limited to, the olfactory tubercle, cerebral cortex ( frontal and entorhinal regions), nucleus accumbens, striatum, caudate nucleus, hippocampus, and the molecular layer of the cerebellum. Based on its abundance in extrapyramidal, limbic, and cortical regions it can be suggested that the 5HT6 receptor plays a role in functions like motor control, emotionality, cognition, and memory. Function Blockade of central 5HT6 receptors has been shown to increase glutamatergic and cholinergic neurotransmission in various brain areas, whereas activation enh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT1D Receptor
5-hydroxytryptamine (serotonin) receptor 1D, also known as HTR1D, is a 5-HT receptor, but also denotes the human gene encoding it. 5-HT1D acts on the central nervous system, and affects locomotion and anxiety. It also induces vasoconstriction in the brain. Tissue distribution 5HT1D receptors are found at low levels in the basal ganglia (globus pallidus, substantia nigra, caudate putamen), the hippocampus, and in the cortex. Structure 5HT1D receptor is a G protein linked receptor that activates an intracellular messenger cascade to produce an inhibitory response by decreasing cellular levels of cAMP. The 5HT1D is a 7-TM receptor. A large intercellular loop between TM-5 and TM-6 is believed to be associated with coupling to a second messenger. Agonists might bind in a manner that utilizes an aspartate residue in TM-3 and residues in the TM-4, TM-5 and TM-6. A human clone containing an intronless open reading frame was found to encode 377 amino acids of the 5HT1D receptor. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin
Serotonin (), also known as 5-hydroxytryptamine (5-HT), is a monoamine neurotransmitter with a wide range of functions in both the central nervous system (CNS) and also peripheral tissues. It is involved in mood, cognition, reward, learning, memory, and physiological processes such as vomiting and vasoconstriction. In the CNS, serotonin regulates mood, appetite, and sleep. Most of the body's serotonin—about 90%—is synthesized in the gastrointestinal tract by enterochromaffin cells, where it regulates intestinal movements. It is also produced in smaller amounts in the brainstem's raphe nuclei, the skin's Merkel cells, pulmonary neuroendocrine cells, and taste receptor cells of the tongue. Once secreted, serotonin is taken up by platelets in the blood, which release it during clotting to promote vasoconstriction and platelet aggregation. Around 8% of the body's serotonin is stored in platelets, and 1–2% is found in the CNS. Serotonin acts as both a vasoconstrictor and vas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convulsant
A convulsant is a drug which induces convulsions or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to poor therapeutic indices. Most convulsants are antagonists (or inverse agonists) at either the GABAA or/and glycine receptors (e.g the pesticide fipronil), or ionotropic glutamate receptor agonists (e.g the marine toxin domoic acid). Many other drugs may cause convulsions as a side effect at high doses (e.g. bupropion, tramadol, pethidine, dextropropoxyphene, clomipramine) but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
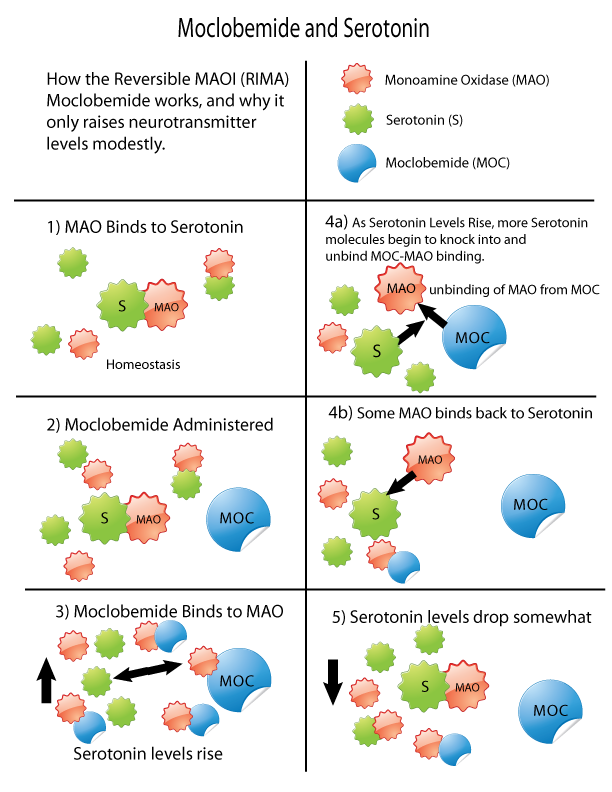
Monoamine Oxidase Inhibition
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with agoraphobia, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |