|
Sulfonyl Halides
In chemistry, a sulfonyl halide consists of a sulfonyl () functional group, group Single bond, singly bonded to a halogen atom. They have the general Chemical formula, formula , where X is a halogen. The Chemical stability, stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series. Sulfonyl halides have tetrahedral sulfur centres attached to two oxygen atoms, an organic radical, and a halide. In a representative example, methanesulfonyl chloride, the S=O, S−C, and S−Cl bond distances are respectively 142.4, 176.3, and 204.6 pm. Sulfonyl chlorides Sulfonic acid chlorides, or sulfonyl chlorides, are a sulfonyl halide with the general formula . Production Arylsulfonyl chlorides are made industrially in a two-step, one-pot reaction from an arene (in this case, benzene) and chlorosulfuric acid: : : The intermediate benzenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thionyl Chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately Volatility (chemistry), volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a Halogenation, chlorinating reagent, with approximately per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also List of Schedule 3 substances (CWC), listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons. Thionyl chloride is sometimes confused with sulfuryl chloride, , but the properties of these compounds differ significantly. Sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. Production The major industrial synthesis involves the reaction of sulfur trioxide and sulfur dichloride. This synthesis can be adapted to the laboratory by heating oleum to slowly distill the sulfur trioxide into a cooled fla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesyl Chloride
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula . Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group –, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene (methylenedioxosulfur(VI)).Valerie Vaillancourt, Michele M. Cudahy, Matthew M. Kreilein and Danielle L. Jacobs "Methanesulfonyl Chloride" in E-EROS Encyclopedia for Reagents in Organic Synthesis Preparation It is produced by the reaction of methane and sulfuryl chloride in a radical reaction: : Another method of production entails chlorination of methanesulfonic acid with thionyl chloride or phosgene: : : Reactions Methanesulfonyl chloride is a precursor to many compounds because it is highly re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
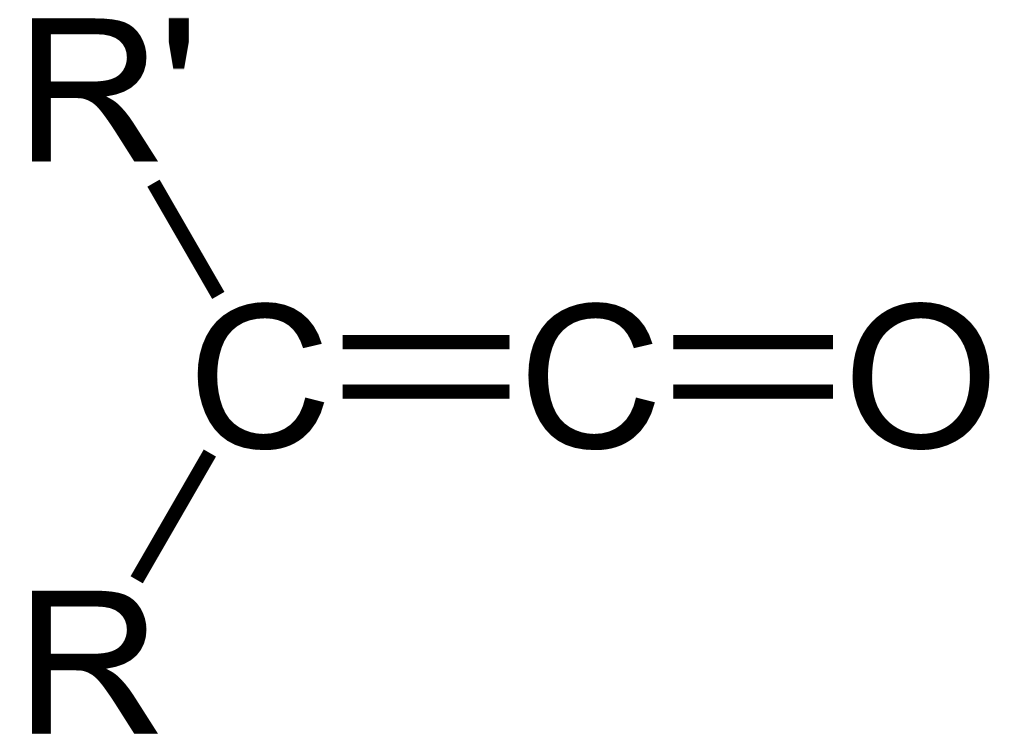
Ketene Acetal
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are highly electrophilic at the carbon atom bonded with the heteroatom, d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenesulfonyl Chloride
Benzenesulfonyl chloride is an organosulfur compound with the formula C6H5SO2Cl. It is a colourless viscous oil that dissolves in organic solvents, but reacts with compounds containing reactive N-H and O-H bonds. It is mainly used to prepare sulfonamides and sulfonate esters by reactions with amines and alcohols, respectively. The closely related compound toluenesulfonyl chloride is often preferred analogue because it is a solid at room temperature and easier to handle. Production The compound is prepared by the chlorsulfonation of benzene: : Benzenesulfonic acid is an intermediate in this conversion. Diphenylsulfone is a side product. Benzenesulfonyl chloride can also be prepared by treating benzenesulfonate salts with phosphorus oxychloride. Reactions Benzenesulfonyl chloride is an electrophilic reagent. It hydrolyzes with heat but is stable toward cold water. Amines react to give sulfonamides. This reaction is the basis of the Hinsberg test for amine In chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
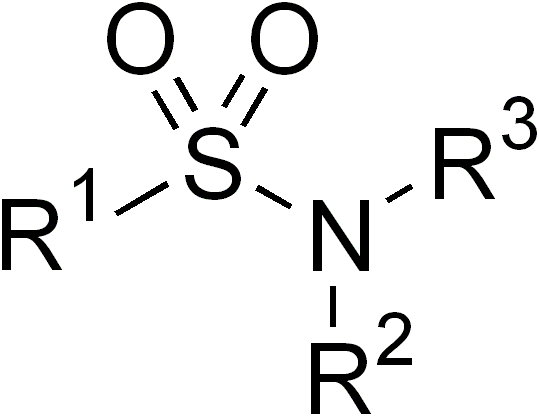
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for Sulfonamide (m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hinsberg Reaction
The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary amines. The reaction was first described by Oscar Hinsberg in 1890. In this test, the amine is shaken well with the Hinsberg reagent ( benzenesulfonyl chloride) in the presence of aqueous alkali (either KOH or NaOH). A primary amine will form a soluble sulfonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulfonamide. A tertiary amine will not react with the original reagent (benzene sulfonyl chloride) and will remain insoluble. After adding dilute acid this insoluble amine is converted to a soluble ammonium salt. In this way the reaction can distinguish between the three types of amines. Tertiary amines are able to react with benzenesulfonyl chloride under a variety of conditions; the test described above is not absolute. The Hinsberg test for amines is valid only when re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: : In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. In a related process, carboxyli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reed Reaction
The Reed reaction is a chemical reaction that utilizes light to oxidize hydrocarbons to alkyl sulfonyl chlorides. This reaction is employed in modifying polyethylene to give chlorosulfonated polyethylene (CSPE), which is noted for its toughness. Commercial implementations Polyethylene is treated with a mixture of chlorine and sulfur dioxide under UV-radiation. Vinylsulfonic acid can also be prepared beginning with the sulfochlorination of chloroethane. Dehydrohalogenation of the product gives vinylsulfonyl chloride, which subsequently is hydrolyzed to give vinylsulfonic acid: :: ::= :: Mechanism The reaction occurs via a free radical mechanism. UV-light initiates homolysis of chlorine, producing a pair of chlorine atoms: Chain initiation: ::::Cl2 -> \nu2Cl. Thereafter a chlorine atom attacks the hydrocarbon chain, freeing hydrogen to form hydrogen chloride and an alkyl free radical. The resulting radical then captures SO2. The resulting sulfonyl radical attack ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2). History Copper(I) chloride was first prepared by Robert Boyle and designated rosin of copper in the mid-seventeenth century from mercury(II) chloride ("Venetian sublimate") and copper metal: :HgCl2 + 2 Cu → 2 CuCl + Hg In 1799, Joseph Proust first differentiated two different chlorides of copper. He prepared CuCl (which he called white muriate of copper) by heating CuCl2 at red heat in the absence of air, causing it to lose half of its combined chlorine followed by removing residual CuCl2 by washing with water. An acidic solution of CuCl was formerly used to analyze carbon monoxide content in gases, for example in Hempel's gas apparatus where the CuCl absorbs the carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |