|
Siderophores
Siderophores (Greek: "iron carrier") are small, high-affinity iron-Chelation, chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated, siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants. Scarcity of soluble iron Despite being one of the most abundant elements in the Earth's crust, iron is not readily bioavailable. In most aerobic environments, such as the soil or sea, iron exists in the ferric (Fe3+) state, which tends to form insoluble rust-like solids. To be effective, nutrients must not only be available, they must be soluble. Microbes release siderophores to scavenge iron from these mineral phases by formation of soluble Fe3+ Complex (chemistry), complexes that can be taken up by active transport mechanisms. Many siderophores are nonribosomal peptides, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siderocalin
Siderocalin (Scn), lipocalin-2, NGAL, 24p3 is a mammalian lipocalin-type protein that can prevent iron acquisition by pathogenic bacteria by binding siderophores, which are iron-binding chelators made by microorganisms. Iron serves as a key nutrient in host (biology), host-pathogen interactions, and pathogens can acquire iron from the host organism via Biosynthesis, synthesis and release siderophores such as enterobactin. Siderocalin is a part of the mammalian defence mechanism and acts as an antibacterial agent. Crystallographic studies of Scn demonstrated that it includes a wikt:calyx, calyx, a ligand-binding domain that is lined with polar cationic groups. Central to the siderophore/siderocalin recognition mechanism are hybrid electrostatic/cation-pi interactions. To evade the host defences, pathogens evolved to produce structurally varied siderophores that would not be recognized by siderocalin, allowing the bacteria to acquire iron. Iron requirements of host organisms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
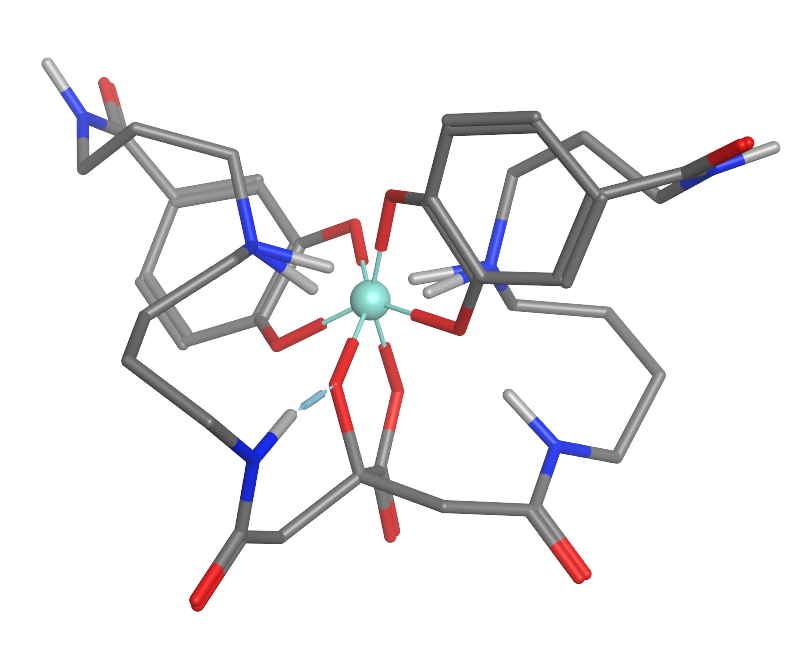
Enterobactin
Enterobactin (also known as enterochelin) is a high affinity siderophore that acquires iron for microbial systems. It is primarily found in Gram-negative bacteria, such as ''Escherichia coli'' and ''Salmonella typhimurium''. Enterobactin is the strongest siderophore known, binding to the ferric ion (Fe3+) with affinity K = 1052 M−1. This value is substantially larger than even some synthetic metal chelators, such as EDTA (Kf,Fe3+ ~ 1025 M−1). Due to its high affinity, enterobactin is capable of chelating even in environments where the concentration of ferric ion is held very low, such as within living organisms. Pathogenic bacteria can steal iron from other living organisms using this mechanism, even though the concentration of iron is kept extremely low due to the toxicity of free iron. Structure and biosynthesis Chorismic acid, an aromatic amino acid precursor, is converted to 2,3-dihydroxybenzoic acid (DHB) by a series of enzymes, EntA, EntB and EntC. An amide linkage of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deferoxamine
Deferoxamine (DFOA), also known as desferrioxamine and sold under the brand name Desferal, is a medication that binds iron and aluminium. It is specifically used in iron overdose, hemochromatosis either due to multiple blood transfusions or an underlying genetic condition, and aluminium toxicity in people on dialysis. It is used by injection into a muscle, vein, or under the skin. Common side effects include pain at the site of injection, diarrhea, vomiting, fever, hearing loss, and eye problems. Severe allergic reactions including anaphylaxis and low blood pressure may occur. It is unclear if use during pregnancy or breastfeeding is safe for the baby. Deferoxamine is a siderophore from the bacteria '' Streptomyces pilosus''. Deferoxamine was approved for medical use in the United States in 1968. It is on the World Health Organization's List of Essential Medicines. Medical uses Deferoxamine is used to treat acute iron poisoning, especially in small children. This agent i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Petrobactin
Petrobactin is a bis-catechol siderophore found in Marinobacter hydrocarbonoclasticus, M. hydrocarbonoclasticus, Alteromonas macleodii, A. macleodii, and the anthrax-producing Bacillus anthracis, B. anthracis. Like other siderophores petrobactin is a highly specific iron(III) transport ligand, contributing to the Siderophore#Marine water, marine microbial uptake of environmental iron. The iron-Chelation, chelated petrobactin complex readily undergoes a Photodissociation, photolytic oxidative decarboxylation due to its α-hydroxy carboxylate group, converting iron(III) to the more biologically useful iron(II). Biological function Like other siderophores, petrobactin is secreted by an animal pathogenic bacterium. B. anthracis uses petrobactin to acquire iron from its host. Interestingly, while the 3,4-catecholate ends of petrobactin do not improve iron(III) affinity relative to hydroxamate ends, they speed up iron removal from human diferric transferrin. Petrobactin in its fe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |