Petrobactin on:
[Wikipedia]
[Google]
[Amazon]
Petrobactin is a bis-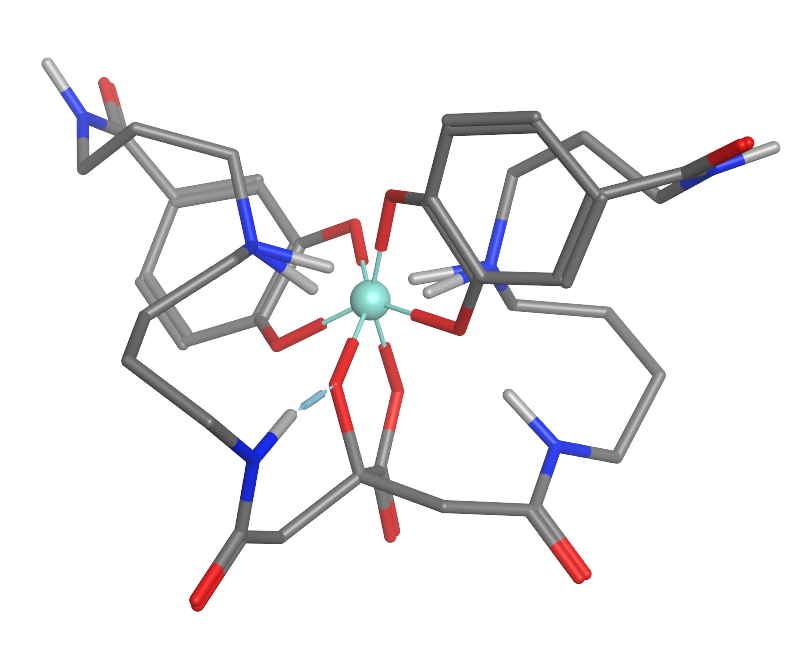
 If the enzymation reactions in this pathway proceed generally, in domains AsbA and AsbB the
If the enzymation reactions in this pathway proceed generally, in domains AsbA and AsbB the
catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is an organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amounts. It ...
siderophore
Siderophores (Greek: "iron carrier") are small, high-affinity iron- chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is n ...
found in M. hydrocarbonoclasticus, A. macleodii, and the anthrax
Anthrax is an infection caused by the bacterium '' Bacillus anthracis'' or ''Bacillus cereus'' biovar ''anthracis''. Infection typically occurs by contact with the skin, inhalation, or intestinal absorption. Symptom onset occurs between one ...
-producing B. anthracis. Like other siderophores petrobactin is a highly specific iron(III)
In chemistry, iron(III) or ''ferric'' refers to the element iron in its +3 oxidation state. ''Ferric chloride'' is an alternative name for iron(III) chloride (). The adjective ''ferrous'' is used instead for iron(II) salts, containing the catio ...
transport ligand, contributing to the marine microbial uptake of environmental iron.
The iron-chelated
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
petrobactin complex readily undergoes a photolytic oxidative decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
due to its α-hydroxy carboxylate group, converting iron(III) to the more biologically useful iron(II)
In chemistry, iron(II) refers to the element iron in its +2 oxidation state. The adjective ''ferrous'' or the prefix ''ferro-'' is often used to specify such compounds, as in ''ferrous chloride'' for iron(II) chloride (). The adjective ''ferr ...
.

Biological function
Like other siderophores, petrobactin is secreted by an animal pathogenic bacterium. B. anthracis uses petrobactin to acquire iron from its host. Interestingly, while the 3,4-catecholate ends of petrobactin do not improve iron(III) affinity relative to hydroxamate ends, they speed up iron removal from human diferrictransferrin
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Iron(III), Fe3+ ions. Human transferrin is ...
. Petrobactin in its ferric and iron-free forms is bound selectively by YclQ (an isogenic disruption mutant
In biology, and especially in genetics, a mutant is an organism or a new genetic character arising or resulting from an instance of mutation, which is generally an alteration of the DNA sequence of the genome or chromosome of an organism. It i ...
in the transporter encoded by the yclNOPQ operon
In genetics, an operon is a functioning unit of DNA containing a cluster of genes under the control of a single promoter. The genes are transcribed together into an mRNA strand and either translated together in the cytoplasm, or undergo splic ...
in Bacillus subtilis
''Bacillus subtilis'' (), known also as the hay bacillus or grass bacillus, is a gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants, humans and marine sponges. As a member of the genus ''Bacill ...
), as is petrobactin's precursor protocatechuic acid
Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in ''in vitro'' and ''in vivo'' studies. It is ...
and the ferric petrobactin photoproduct. The yclNOPQ operon is required for the utilization of petrobactin and yclNOPQ orthologs likely contribute to the pathogenicity of Bacilli
Bacilli is a Taxonomy (biology), taxonomic Class (biology), class of bacteria that includes two orders, Bacillales and Lactobacillales, which contain several well-known pathogens such as ''Bacillus anthracis'' (the cause of anthrax). ''Bacilli'' ...
.
Biosynthesis
In B. anthracis, petrobactin is produced by a nonribosomal peptide synthetase independent siderophore (NIS) synthetase pathway. The enzyme sequences used are anthrax siderophore biosynthesis (Asb) A through F, in alphabetical order. These gene clusters are identical to those used in M. hydrocarbonclasticus biosynthesis of petrobactin. In A. macleodii only the first three gene clusters, AsbA through AsbC, are identical to B. anthracis; then a longer AsbD and AsbF is next, followed by two hypothetical protein domains and a PepSY domain-containing gene. A. macleodii ends its sequence with AsbE. The biosynthesis of petrobactin in B. anthracis can progress in order AsbA-AsbB-AsbE-AsbE or AsbA-AsbE-AsbB-AsbE.phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols:
:
This equation can be writ ...
of a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
forms an acylphosphate intermediate, which is then dephosphorylated by a primary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
in spermidine
Spermidine is a polyamine compound () found in ribosomes and living tissues and having various metabolic functions within organisms.
Function
Spermidine is an Aliphatic compound, aliphatic polyamine. Spermidine synthase (SPDS) catalyzes its form ...
. In domain AsbE the lone pair of electrons on a primary amine allows for a nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
on the electrophilic hydroxyl carbon. The sulfur on AsbE is protonated to form a thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
and the amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
nitrogen is deprotonated.
The dehydration of 3-dehydroshikimic acid might proceed as a modified, enzyme-catalyzed dienol benzene rearrangement and reduction, leading to aromatization of the ring.
References
{{microorganisms Biomolecules Iron metabolism Catechols Carboxamides Polyols Secondary amino acids