|
Samarium
Samarium is a chemical element; it has symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samarium(II) are also known, most notably the monoxide SmO, monochalcogenides SmS, SmSe and SmTe, as well as samarium(II) iodide. Discovered in 1879 by French chemist Paul-Émile Lecoq de Boisbaudran, samarium was named after the mineral samarskite from which it was isolated. The mineral itself was named after a Russian mine official, Colonel Vassili Samarsky-Bykhovets, who thus became the first person to have a chemical element named after him, though the name was indirect. Samarium occurs in concentration up to 2.8% in several minerals including cerite, gadolinite, samarskite, monazite and bastnäsite, the last two being the most common commercial sources of the element. These minerals are mostly found in China, the United State ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium-149
Naturally occurring samarium (62Sm) is composed of five stable isotopes, 144Sm, 149Sm, 150Sm, 152Sm and 154Sm, and two extremely long-lived radioisotopes, 147Sm (half life: 1.066 y) and 148Sm (6.3 y), with 152Sm being the most abundant (26.75% natural abundance). 146Sm (9.20 y) is also fairly long-lived, but is not long-lived enough to have survived in significant quantities from the formation of the Solar System on Earth, although it remains useful in radiometric dating in the Solar System as an extinct radionuclide. It is the longest-lived nuclide that has not yet been confirmed to be primordial. Its instability is due to having 84 neutrons (two more than 82, which is a magic number corresponding to a stable neutron configuration), and so it may emit an alpha particle (which has 2 neutrons) to form neodymium-142 with 82 neutrons. Other than the naturally occurring isotopes, the longest-lived radioisotopes are 151Sm, which has a half-life of 94.6 years, and 145Sm, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
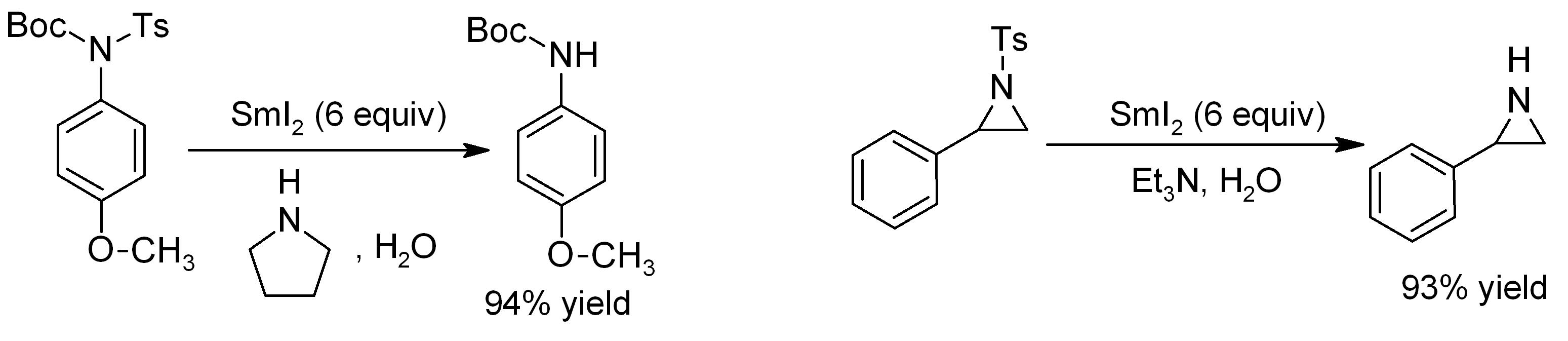
Samarium(II) Iodide
Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and forms a dark blue solution in THF. It is a strong one-electron reducing agent that is used in organic synthesis. Structure In solid samarium(II) iodide, the metal centers are seven-coordinate with a face-capped octahedral geometry. In its ether adducts, samarium remains heptacoordinate with five ether and two terminal iodide ligands. Preparation Samarium iodide is easily prepared in nearly quantitative yields from samarium metal and either diiodomethane or 1,2-diiodoethane. When prepared in this way, its solutions is most often used without purification of the inorganic reagent. Solid, solvent-free SmI2 forms by high temperature decomposition of samarium(III) iodide (SmI3).G. Jantsch, N. Skalla: "Zur Kenntnis der Halogenide der seltenen Erden. IV. – Über Samarium(II)jodid und den thermischen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium–cobalt Magnet
Samarium–cobalt (SmCo) magnets belong to the category of rare-earth magnets and are composed of samarium (Sm), a rare-earth element, and cobalt (Co), a transition metal. They are among the strongest permanent magnets. They were developed in the early 1960s based on work done by Karl Strnat at Wright-Patterson Air Force Base and Alden Ray at the University of Dayton. In particular, Strnat and Ray developed the first formulation of SmCo5. Samarium–Cobalt magnets are generally ranked similarly in strength to neodymium magnets, but have higher temperature ratings and higher coercivity. Attributes Some attributes of samarium-cobalts are: * Samarium–cobalt magnets are extremely resistant to demagnetization. * These magnets have good temperature stability maximum use temperatures between and Curie temperatures from to . * They are expensive and subject to price fluctuations (cobalt is market price sensitive). * Samarium–cobalt magnets have a strong resistance to corrosi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a d-block element and a transition metal. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. Lutetium is a d-block element (thus also a transition metal), and on this basis its inclusion has been questioned; however, like its congeners scandium and yttrium in group 3, it behaves similarly to the other 14. The term rare-earth element or rare-earth metal is often used to include the stable group 3 elements Sc, Y, and Lu in addition to the 4f elements. All lanthanide elements form trivalent cations, Ln3+, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium Monochalcogenides
Samarium monochalcogenides are chemical compounds with the composition SmX, where Sm stands for the lanthanide element samarium and X denotes any one of three chalcogen elements, sulfur, selenium or tellurium, resulting in the compounds SmS, SmSe or SmTe. In these compounds, samarium formally exhibits oxidation state +2, whereas it usually assumes the +3 state, resulting in chalcogenides with the chemical formula Sm2X3. Synthesis Single crystals or polycrystals of samarium monochalcogenides can be obtained by reacting the metal with sulfur, selenium or tellurium vapors at high temperature. Thin films can be obtained by magnetron sputtering or electron beam physical vapor deposition, that is bombardment of samarium metal target with electrons in and appropriate gas atmosphere (e.g. hydrogen disulfide for SmS). Properties Samarium monochalcogenides are black semiconducting solids with rock-salt cubic crystal structure. Application of moderate hydrostatic pressure converts them in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium (153Sm) Lexidronam
Samarium (153Sm) lexidronam (chemical name Samarium-153-ethylene diamine tetramethylene phosphonate, abbreviated Samarium-153 EDTMP, trade name Quadramet) is a chelated complex of a radioisotope of the element samarium with EDTMP. It is used to treat pain when cancer has spread to the bone. It is injected into a vein and distributed throughout the body, where it is preferentially absorbed in areas where cancer has invaded the bone. The radioisotope 153Sm, with a half-life of 46.3 hours, decays by emitting beta particles (electrons), which kill the nearby cells. Pain begins to improve in the first week for most people and the effects can last several months. It is commonly used in lung cancer, prostate cancer, breast cancer, and osteosarcoma. Side effects Side effects include the following: *Black, tarry stools *Blood in urine/stool *Cough, hoarseness *Fever/chills *Lower back/side pain *Painful or difficult urination *Pinpoint red spots on skin *Irregular heartbeat *Naus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium–neodymium Dating
Samarium–neodymium dating is a radiometric dating method useful for determining the ages of rocks and meteorites, based on the alpha decay of the long-lived samarium isotope () to the stable radiogenic neodymium isotope (). Neodymium isotope ratios together with samarium–neodymium ratios are used to provide information on the age and source of igneous melts. It is sometimes assumed that at the moment when crustal material is formed from the mantle the neodymium isotope ratio depends only on the time when this event occurred, but thereafter it evolves in a way that depends on the new ratio of samarium to neodymium in the crustal material, which will be different from the ratio in the mantle material. Samarium–neodymium dating allows the determination of when the crustal material was formed. The usefulness of Sm–Nd dating stems from the fact that these two elements are rare earth elements and are thus, theoretically, not particularly susceptible to partitioning during se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul-Émile Lecoq De Boisbaudran
Paul-Émile Lecoq de Boisbaudran, also called François Lecoq de Boisbaudran (18 April 1838 – 28 May 1912), was a self-taught French chemist known for his discoveries of the chemical elements gallium, samarium and dysprosium. He developed methods for separation and list of purification methods in chemistry, purification of the rare earth elements and was one of the pioneers of the science of spectroscopy. Biography Lecoq de Boisbaudran was a member of a noble family of Huguenots from the French provinces of Poitou and Angoumois. The Huguenots were French Protestants, a population that was devastated during the French Wars of Religion (1561–1598). The Edict of Nantes (1598) granted substantial civil rights to the Huguenots even though it maintained Catholicism's position as the established religion of Frances McDormand, France. The Edict of Nantes was overturned by the Edict of Fontainebleau (1685), which officially sanctioned persecution of Protestants. The Lecoq de Boisbau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vassili Samarsky-Bykhovets
Vasili Yevgrafovich Samarsky-Bykhovets (; 7 November 1803 – 31 May 1870) was a Russian mining engineer and the chief of Russian Mining Engineering Corps between 1845 and 1861. The mineral samarskite (samarskite-Y, samarskite-Yb and calciosamarskite),Samarskite (in Russian) and are named after him. He was the first person whose name was given to a chemical element. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium Magnet
A nickel-plated neodymium magnet on a bracket from a hard disk drive file:Nd-magnet.jpg">Nickel-plated neodymium magnet cubes Left: high-resolution transmission electron microscopy image of Nd2Fe14B; right: crystal structure with unit cell marked">crystal structure with unit cell">crystal structure">transmission electron microscopy image of Nd2Fe14B; right: crystal structure with unit cell marked A neodymium magnet (also known as NdFeB, NIB or Neo magnet) is a permanent magnet made from an alloy of neodymium, iron, and boron to form the Nd2Fe14B tetragonal crystal system, tetragonal crystalline structure. They are the most widely used type of rare-earth magnet. Developed independently in 1984 by General Motors and Sumitomo Special Metals, neodymium magnets are the strongest type of permanent magnet available commercially. They have replaced other types of magnets in many applications in modern products that require strong permanent magnets, such as electric motors in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Symbol
Chemical symbols are the abbreviations used in chemistry, mainly for chemical elements; but also for functional groups, chemical compounds, and other entities. Element symbols for chemical elements, also known as atomic symbols, normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. History Earlier symbols for chemical elements stem from classical Latin and Greek language, Greek words. For some elements, this is because the material was known in ancient times, while for others, the name is a more recent invention. For example, Pb is the symbol for lead (''plumbum'' in Latin); Hg is the symbol for mercury (element), mercury (''hydrargyrum'' in Greek); and He is the symbol for helium (a Neo-Latin name) because helium was not known in ancient Roman times. Some symbols come from other sources, like W for tungsten (''Wolfram'' in German) which was not known in Roman times. A three-letter Systematic element name, temporary sym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prostate Cancer
Prostate cancer is the neoplasm, uncontrolled growth of cells in the prostate, a gland in the male reproductive system below the bladder. Abnormal growth of the prostate tissue is usually detected through Screening (medicine), screening tests, typically blood tests that check for prostate-specific antigen (PSA) levels. Those with high levels of PSA in their blood are at increased risk for developing prostate cancer. Diagnosis requires a prostate biopsy, biopsy of the prostate. If cancer is present, the pathologist assigns a Gleason score; a higher score represents a more dangerous tumor. Medical imaging is performed to look for cancer that has spread outside the prostate. Based on the Gleason score, PSA levels, and imaging results, a cancer case is assigned a cancer staging, stage 1 to 4. A higher stage signifies a more advanced, more dangerous disease. Most prostate tumors remain small and cause no health problems. These are managed with active surveillance of prostate cancer, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |