|
Nitrosoureas
Nitrosourea is both the name of a molecule, and a class of compounds that include a nitroso (R-NO) group and a urea. Examples Examples include: * Arabinopyranosyl-N-methyl-N-nitrosourea, Arabinopyranosyl-''N''-methyl-''N''-nitrosourea (Aranose) * Carmustine (BCNU, BiCNU) * Chlorozotocin * Ethylnitrosourea (ENU) * Fotemustine * Lomustine (CCNU) * Nimustine * N-Nitroso-N-methylurea, ''N''-Nitroso-''N''-methylurea (NMU) * Ranimustine (MCNU) * Semustine * Streptozocin (Streptozotocin) Nitrosourea compounds are DNA alkylating antineoplastic agent, alkylating agents and are often used in chemotherapy. They are lipophile, lipophilic and thus can cross the blood–brain barrier, making them useful in the treatment of brain tumors such as glioblastoma multiforme. File:Aranose (Haworth).svg, Arabinopyranosyl-N-methyl-N-nitrosourea, Arabinopyranosyl-''N''-methyl-''N''-nitrosourea File:Carmustine.svg, Carmustine File:Chlorozotocin (Haworth).svg, Chlorozotocin File:ENU.svg, Ethylnitrosourea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arabinopyranosyl-N-methyl-N-nitrosourea
Arabinopyranosyl-''N''-methyl-''N''-nitrosourea, also known as Aranose (Араноза) is a cytostatic cancer, anticancer chemotherapy (oncology), chemotherapeutic drug of an alkylating antineoplastic agent, alkylating type. Chemically it is a nitrosourea derivative. It was developed in the Soviet Union in the 1970s. It was claimed by its developers that its advantages over other nitrosoureas are a relatively low hematological toxicity (compared to other nitrosoureas available at that time) and a wider therapeutic index, which allows for its outpatient administration. History It was first synthesized in late 1970s in the Laboratory of Organic Synthesis of Soviet Cancer Research Institute (which belonged to Academy of Medical Sciences of the USSR). Its first clinical trials in USSR were conducted in the late 1980s. Those trials confirmed its potential clinical efficacy in melanoma and better relative safety & improved tolerability over other nitrosourea antineoplastic compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lomustine
Lomustine ( INN; abbreviated as CCNU; original brand name CeeNU, now marketed as Gleostine) is an alkylating nitrosourea compound used in chemotherapy. It is closely related to semustine and is in the same family as streptozotocin. It is a highly lipid-soluble drug, thus it crosses the blood–brain barrier. This property makes it ideal for treating brain tumors, which is its primary use, although it is also used to treat Hodgkin lymphoma as a second-line option. It has also been used in veterinary practice as a treatment for cancers in cats and dogs. Lomustine is a bifunctional alkylating agent, alkylates both DNA and RNA, has the ability to created interstrand cross-links (ICLs) in DNA. As with other nitrosoureas, it may also inhibit several key enzymatic processes by carbamoylation of amino acids in proteins. Lomustine is cell-cycle nonspecific. Medical uses Chemotherapy in human medicine Lomustine is an alkylating chemotherapy drug that is indicated by the FDA for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemotherapy
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs (list of chemotherapeutic agents, chemotherapeutic agents or alkylating agents) in a standard chemotherapy regimen, regimen. Chemotherapy may be given with a cure, curative intent (which almost always involves combinations of drugs), or it may aim only to prolong life or to Palliative care, reduce symptoms (Palliative care, palliative chemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called ''oncology#Specialties, medical oncology''. The term ''chemotherapy'' now means the non-specific use of intracellular poisons to inhibit mitosis (cell division) or to induce DNA damage (naturally occurring), DNA damage (so that DNA repair can augment chemotherapy). This meaning excludes the more-selective agents that block extracellular signals (signal transduction) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroso
In organic chemistry, nitroso refers to a functional group in which the nitric oxide () group is attached to an organic moiety. As such, various nitroso groups can be categorized as ''C''-nitroso compounds (e.g., nitrosoalkanes; ), ''S''-nitroso compounds ( nitrosothiols; ), ''N''-nitroso compounds (e.g., nitrosamines, ), and ''O''-nitroso compounds ( alkyl nitrites; ). Synthesis Nitroso compounds can be prepared by the reduction of nitro compounds or by the oxidation of hydroxylamines. Ortho-nitrosophenols may be produced by the Baudisch reaction. In the Fischer–Hepp rearrangement, aromatic 4-nitrosoanilines are prepared from the corresponding nitrosamines. Properties Nitrosoarenes typically participate in a monomer–dimer equilibrium. The azobenzene ''N'',''N'-''dioxide (Ar(–O)N+=+N(O–)Ar) dimers, which are often pale yellow, are generally favored in the solid state, whereas the deep-green monomers are favored in dilute solution or at higher temperatures. They ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptozocin
Streptozotocin or streptozocin (INN, USP) (STZ) is a naturally occurring alkylating antineoplastic agent that is particularly toxic to the insulin-producing beta cells of the pancreas in mammals. It is used in medicine for treating certain cancers of the islets of Langerhans and used in medical research to produce an animal model for hyperglycemia and Alzheimer's in a large dose, as well as type 2 diabetes or type 1 diabetes with multiple low doses. Usage Streptozotocin is approved by the U.S. Food and Drug Administration (FDA) for treating metastatic cancer of the pancreatic islet cells. Since it carries a substantial risk of toxicity and rarely cures the cancer, its use is generally limited to patients whose cancer cannot be removed by surgery. In these patients, streptozotocin can reduce the tumor size and reduce symptoms (especially hypoglycemia due to excessive insulin secretion by insulinomas). A typical dose is 500 mg/m2/day by intravenous injection, for 5 days, rep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
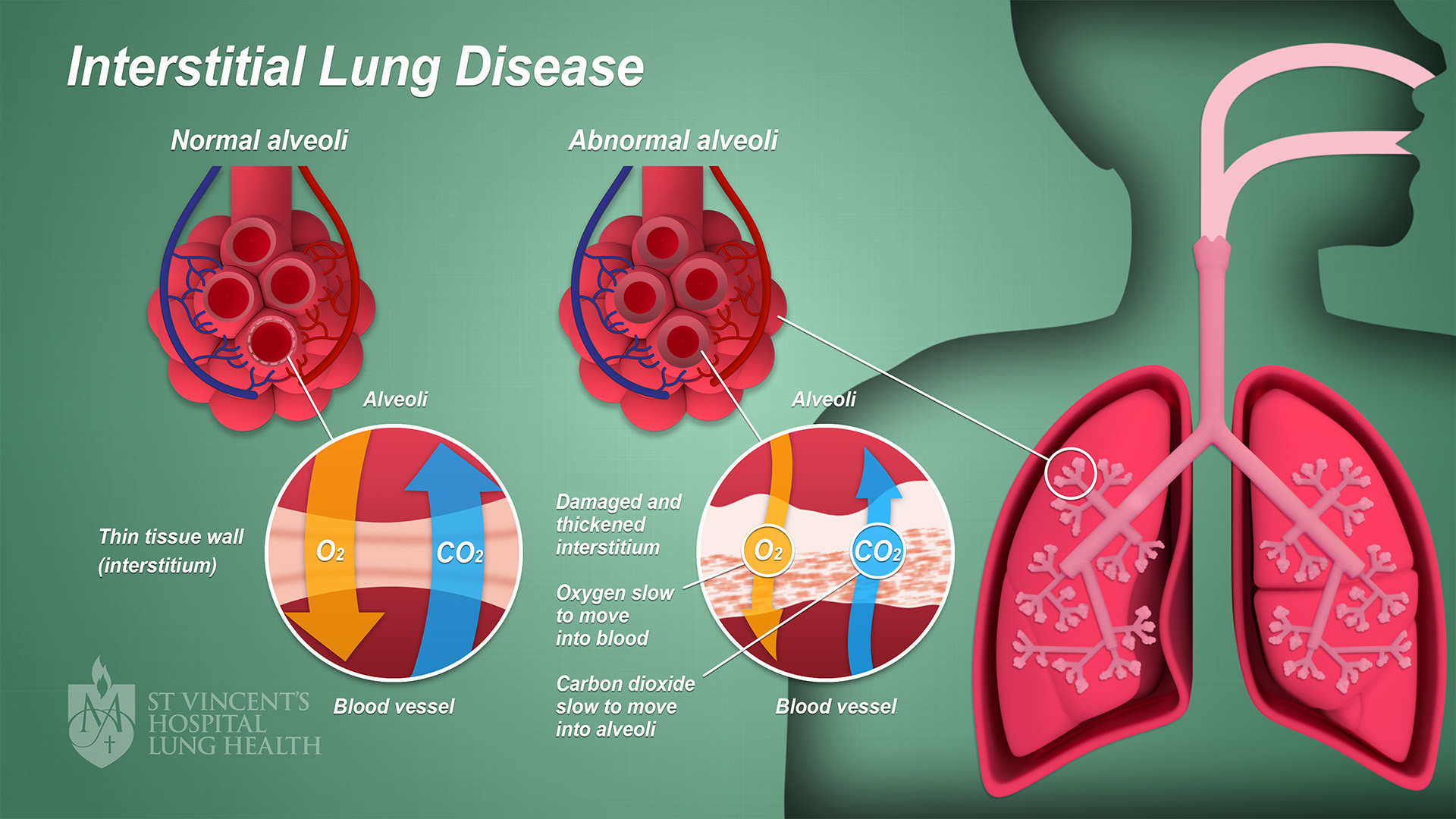
Interstitial Lung Disease
Interstitial lung disease (ILD), or diffuse parenchymal lung disease (DPLD), is a group of respiratory diseases affecting the interstitium (the tissue) and space around the alveoli (air sacs) of the lungs. It concerns alveolar epithelium, pulmonary capillary endothelium, basement membrane, and perivascular and perilymphatic tissues. It may occur when an injury to the lungs triggers an abnormal healing response. Ordinarily, the body generates just the right amount of tissue to repair damage, but in interstitial lung disease, the repair process is disrupted, and the tissue around the air sacs (alveoli) becomes scarred and thickened. This makes it more difficult for oxygen to pass into the bloodstream. The disease presents itself with the following symptoms: shortness of breath, nonproductive coughing, fatigue, and weight loss, which tend to develop slowly, over several months. The average rate of survival for someone with this disease is between three and five years. The term IL ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glioblastoma Multiforme
Glioblastoma, previously known as glioblastoma multiforme (GBM), is the most aggressive and most common type of cancer that originates in the brain, and has a very poor prognosis for survival. Initial signs and symptoms of glioblastoma are nonspecific. They may include headaches, personality changes, nausea, and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness. The cause of most cases of glioblastoma is not known. Uncommon risk factors include genetic disorders, such as neurofibromatosis and Li–Fraumeni syndrome, and previous radiation therapy. Glioblastomas represent 15% of all brain tumors. They are thought to arise from astrocytes. The diagnosis typically is made by a combination of a CT scan, MRI scan, and tissue biopsy. There is no known method of preventing the cancer. Treatment usually involves surgery, after which chemotherapy and radiation therapy are used. The medication temozolomide is frequently used as pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brain Tumor
A brain tumor (sometimes referred to as brain cancer) occurs when a group of cells within the Human brain, brain turn cancerous and grow out of control, creating a mass. There are two main types of tumors: malignant (cancerous) tumors and benign tumor, benign (non-cancerous) tumors. These can be further classified as primary tumors, which start within the brain, and metastasis, secondary tumors, which most commonly have spread from tumors located outside the brain, known as brain metastasis tumors. All types of brain tumors may produce symptoms that vary depending on the size of the tumor and the part of the brain that is involved. Where symptoms exist, they may include headaches, seizures, problems with visual perception, vision, vomiting and cognition, mental changes. Other symptoms may include difficulty walking, speaking, with sensations, or unconsciousness. The cause of most brain tumors is unknown, though up to 4% of brain cancers may be caused by CT scan radiation. Uncommo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood–brain Barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that regulates the transfer of solutes and chemicals between the circulatory system and the central nervous system, thus protecting the brain from harmful or unwanted substances in the blood. The blood–brain barrier is formed by endothelial cells of the Capillary, capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive transport, passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function. The blood–brain barrier restricts the passage of pathogens, the diffusion of solutes in the blood, and Molecular mass, large or Hydrophile, hydrophilic molecules into the cerebrospinal fluid, while a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipophile
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are called lipophilic (translated as "fat-loving" or "fat-liking"). Such non-polar solvents are themselves lipophilic, and the adage "like dissolves like" generally holds true. Thus lipophilic substances tend to dissolve in other lipophilic substances, whereas hydrophilic ("water-loving") substances tend to dissolve in water and other hydrophilic substances. Lipophilicity, hydrophobicity, and non-polarity may describe the same tendency towards participation in the London dispersion force, as the terms are often used interchangeably. However, the terms "lipophilic" and "hydrophobic" are not synonymous, as can be seen with silicones and fluorocarbons, which are hydrophobic but not lipophilic. __TOC__ Surfactants Hydrocarbon-based surfactants are compounds that are amphiphilic ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylating Antineoplastic Agent
An alkylating antineoplastic agent is an alkylating agent used in cancer treatment that attaches an alkyl group (CnH2n+1) to DNA. Since cancer cells, in general, proliferate faster and with less error-correcting than healthy cells, cancer cells are more sensitive to DNA damage—such as being alkylated. Alkylating agents are used to treat several cancers. However, they are also toxic to normal cells (cytotoxic), particularly cells that divide frequently, such as those in the gastrointestinal tract, bone marrow, testicles and ovaries, which can cause loss of fertility. Most of the alkylating agents are also carcinogenic. History Before their use in chemotherapy, alkylating agents were better known for their use as sulfur mustard, ("mustard gas") and related chemical weapons in World War I. The nitrogen mustards were the first alkylating agents used medically, as well as the first modern cancer chemotherapies. Goodman, Gilman, and others began studying nitrogen mustards at Yale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ranimustine
Ranimustine ( INN, marketed under the tradename Cymerin; also known as MCNU) is a nitrosourea alkylating agent approved in Japan for the treatment of chronic myelogenous leukemia and polycythemia vera. It has never been filed for FDA evaluation in the United States, where it is not marketed. Synthesis : Ranimustine is made by reacting the primary amine of a pyranose In organic chemistry, pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom (a heterocycle). There may be other carbons external to the ... sugar (2) with ''o''-nitrophenyl N-(2-chloroethyl)-N-nitrosocarbamate (1) to form the nitrosourea group. References External links *Cymerin サイメリン(PDF) Mitsubishi Tanabe Pharma. October 2007. Alkylating antineoplastic agents Nitrosamines Nitrosoureas Ureas Chloroethyl compounds {{antineoplastic-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |