|
Ligases
In biochemistry, a ligase is an enzyme that can catalyze the joining ( ligation) of two molecules by forming a new chemical bond. This is typically via hydrolysis of a small pendant chemical group on one of the molecules, typically resulting in the formation of new C-O, C-S, or C-N bonds. For example, DNA ligase can join two complementary fragments of nucleic acid by forming phosphodiester bonds, and repair single stranded breaks that arise in double stranded DNA during replication. In general, a ligase catalyzes the following dehydration reaction, thus joining molecules A and B: A-OH + B-H → A–B + H2O Nomenclature The naming of ligases is inconsistent and so these enzymes are commonly known by several different names. Generally, the common names of ligases include the word "ligase", such as in DNA ligase, an enzyme commonly used in molecular biology laboratories to join together DNA fragments. However, many common names use the term "synthetase" or "synthase" instead, bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquitin Ligase
A ubiquitin ligase (also called an E3 ubiquitin ligase) is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquitin from the E2 to the protein substrate. In simple and more general terms, the ligase enables movement of ubiquitin from a ubiquitin carrier to another protein (the substrate) by some mechanism. The ubiquitin, once it reaches its destination, ends up being attached by an isopeptide bond to a lysine residue, which is part of the target protein. E3 ligases interact with both the target protein and the E2 enzyme, and so impart substrate specificity to the E2. Commonly, E3s polyubiquitinate their substrate with Lys48-linked chains of ubiquitin, targeting the substrate for destruction by the proteasome. However, many other types of linkages are possible and alter a protein's activity, interactions, or localization. Ubiquitination by E3 ligases re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelatase
In biochemistry, chelatases are enzymes that catalyze the insertion ("metalation") of naturally occurring tetrapyrroles. Many tetrapyrrole-based cofactors exist in nature including hemes, chlorophylls, and vitamin B12. These metallo cofactors are derived by the reaction of metal cations with tetrapyrroles, which are not ligands ''per se'', but the conjugate acids thereof. In the case of ferrochelatases, the reaction that chelatases catalyze is: :Fe2+ + H2P → FeP + 2 H+ In the above equation H2P represents a sirohydrochlorin or a porphyrin, such as protoporphyrin IX. Chelatases are required because porphyrins and related macrocyclic ligands are extremely slow to metalate, despite favorable thermodynamics. These low rates are attributed to the tight fit of the metal into the rigid 18- or 17-membered tetrapyrrole macrocycle. Several families of chelatase are known including cobalt chelatase, magnesium chelatase, and ferrochelatase. Nickel insertion into a sirohydrochlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthase
In biochemistry, a synthase is an enzyme that catalyses a synthesis process. Note that, originally, biochemical nomenclature distinguished synthetases and synthases. Under the original definition, synthases do not use energy from nucleoside triphosphates (such as ATP, GTP, CTP, TTP, and UTP), whereas synthetases do use nucleoside triphosphates. However, the Joint Commission on Biochemical Nomenclature (JCBN) dictates that 'synthase' can be used with any enzyme that catalyzes synthesis (whether or not it uses nucleoside triphosphates), whereas 'synthetase' is to be used synonymously with ' ligase'. Examples * ATP synthase * Citrate synthase * Tryptophan synthase * Pseudouridine synthase * Fatty acid synthase Fatty acid synthase (FAS) is an enzyme that in humans is encoded by the ''FASN'' gene. Fatty acid synthase is a multi-enzyme protein that catalyzes fatty acid synthesis. It is not a single enzyme but a whole enzymatic system composed of two ide ... * Cell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argininosuccinate Synthetase
Argininosuccinate synthase or synthetase (ASS; ) is an enzyme that catalyzes the synthesis of argininosuccinic acid, argininosuccinate from citrulline and aspartic acid, aspartate. In humans, argininosuccinate synthase is encoded by the ''ASS (gene), ASS gene'' located on chromosome 9 (human), chromosome 9. ASS is responsible for the third step of the urea cycle and one of the reactions of the citrulline-NO cycle. Expression The expressed ASS gene is at least 65 kb in length, including at least 12 introns. In humans, ''ASS'' is expressed mostly in the cells of the liver and kidney. Mechanism In the first step of the catalyzed reaction, citrulline attacks the α-phosphate of Adenosine triphosphate, ATP to form citrulline adenylate, a reactive intermediate. The attachment of Adenosine monophosphate, AMP to the ureido (urea-like) group on citrulline activates the carbonyl center for subsequent nucleophilic attack. This activation facilitates the second step, in which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylation
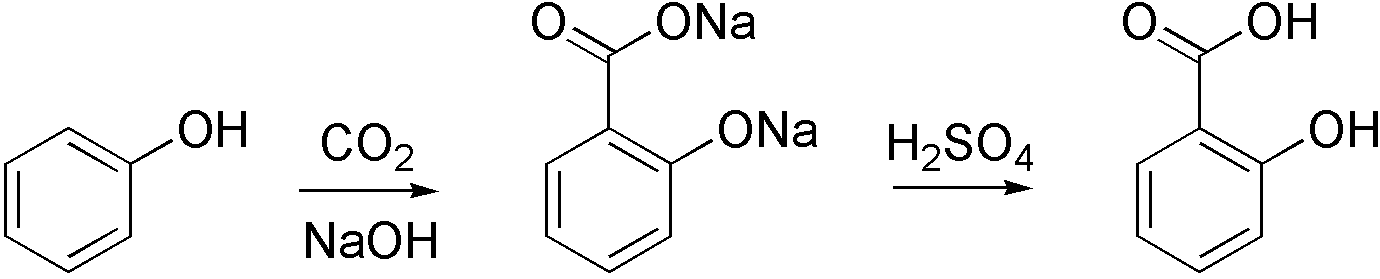
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Ligase
DNA ligase is a type of enzyme that facilitates the joining of DNA strands together by catalyzing the formation of a phosphodiester bond. It plays a role in repairing single-strand breaks in duplex DNA in living organisms, but some forms (such as DNA ligase IV) may specifically repair double-strand breaks (i.e. a break in both complementary strands of DNA). Single-strand breaks are repaired by DNA ligase using the complementary strand of the double helix as a template, with DNA ligase creating the final phosphodiester bond to fully repair the DNA. DNA ligase is used in both DNA repair and DNA replication (see '' Mammalian ligases''). In addition, DNA ligase has extensive use in molecular biology laboratories for recombinant DNA experiments (see '' Research applications''). Purified DNA ligase is used in gene cloning to join DNA molecules together to form recombinant DNA. Enzymatic mechanism The mechanism of DNA ligase is to form two covalent phosphodiester bonds between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-ase
The suffix -ase is used in biochemistry to form names of enzymes. The most common way to name enzymes is to add this suffix onto the end of the substrate, ''e.g.'' an enzyme that breaks down peroxides may be called peroxidase; the enzyme that produces telomeres is called telomerase. Sometimes enzymes are named for the function they perform, rather than substrate, e.g. the enzyme that polymerizes (assembles) DNA into strands is called polymerase; see also reverse transcriptase. Etymology The ''-ase'' suffix is a libfix derived from " diastase", the first recognized enzyme. Its usage in subsequently discovered enzymes was proposed by Émile Duclaux, with the intention of honoring the first scientists to isolate diastase. See also *Amylase *DNA polymerase A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually wo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latin Language
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area around Rome, Italy. Through the expansion of the Roman Republic, it became the dominant language in the Italian Peninsula and subsequently throughout the Roman Empire. It has greatly influenced many languages, Latin influence in English, including English, having contributed List of Latin words with English derivatives, many words to the English lexicon, particularly after the Christianity in Anglo-Saxon England, Christianization of the Anglo-Saxons and the Norman Conquest. Latin Root (linguistics), roots appear frequently in the technical vocabulary used by fields such as theology, List of Latin and Greek words commonly used in systematic names, the sciences, List of medical roots, suffixes and prefixes, medicine, and List of Latin legal terms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peripheral Membrane Protein
Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins. The reversible attachment of proteins to biological membranes has shown to regulate cell signaling and many other important cellular events, through a variety of mechanisms. For example, the close association between many enz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA Carboxylase
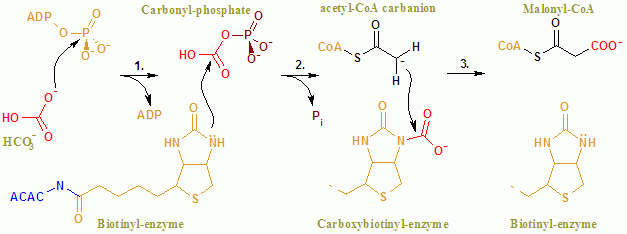
Acetyl-CoA carboxylase (ACC) is a biotin-dependent enzyme () that catalyzes the irreversible carboxylation of acetyl-CoA to produce malonyl-CoA through its two catalytic activities, biotin carboxylase (BC) and carboxyltransferase (CT). ACC is a multi-subunit enzyme in most prokaryotes and in the chloroplasts of most plants and algae, whereas it is a large, multi-domain enzyme in the cytoplasm of most eukaryotes. The most important function of ACC is to provide the malonyl-CoA substrate for the biosynthesis of fatty acids. The activity of ACC can be controlled at the transcriptional level as well as by small molecule modulators and covalent modification. The human genome contains the genes for two different ACCs—'' ACACA'' and '' ACACB''. Structure Prokaryotes and plants have multi-subunit ACCs composed of several polypeptides. Biotin carboxylase (BC) activity, biotin carboxyl carrier protein (BCCP), and carboxyl transferase (CT) activity are each contained on a differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |