|
Design Of Experiments
The design of experiments (DOE), also known as experiment design or experimental design, is the design of any task that aims to describe and explain the variation of information under conditions that are hypothesized to reflect the variation. The term is generally associated with experiments in which the design introduces conditions that directly affect the variation, but may also refer to the design of quasi-experiments, in which natural conditions that influence the variation are selected for observation. In its simplest form, an experiment aims at predicting the outcome by introducing a change of the preconditions, which is represented by one or more independent variables, also referred to as "input variables" or "predictor variables." The change in one or more independent variables is generally hypothesized to result in a change in one or more dependent variables, also referred to as "output variables" or "response variables." The experimental design may also identify ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Response Surface Metodology
Response may refer to: *Call and response (music), musical structure *Reaction (other) *Request–response **Output (computing), Output or response, the result of telecommunications input *Response (liturgy), a line answering a versicle *Response (music) or antiphon, a response to a psalm or other part of a religious service *Response, a phase in emergency management *Response rate (survey) Proper names and titles *''Response'', a print and online magazine of Christian thought published by Seattle Pacific University *Response (album), ''Response'' (album), a studio album by Phil Wickham *Response (company), a call centre company based in Scotland *The Response (film), ''The Response'' (film) *The National War Memorial (Canada), titled ''The Response'' *The Northumberland Fusiliers Memorial in Newcastle upon Tyne, titled "The Response" See also *Action (other) *Answer (other) *Reply (other) *Response variable, or the realization thereof *Res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
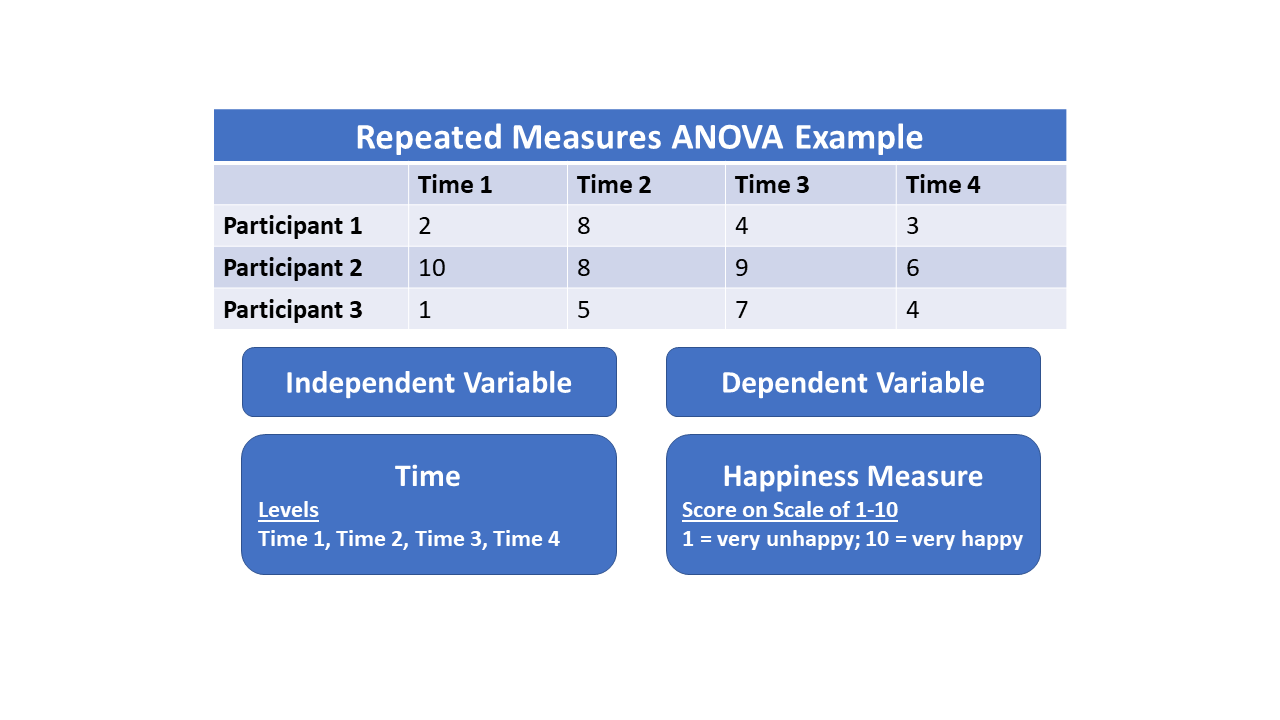
Repeated Measures Design
Repeated measures design is a research design that involves multiple measures of the same variable taken on the same or matched subjects either under different conditions or over two or more time periods. For instance, repeated measurements are collected in a longitudinal study in which change over time is assessed. Crossover studies A popular repeated-measures design is the crossover study. A crossover study is a longitudinal study in which subjects receive a sequence of different treatments (or exposures). While crossover studies can be observational studies, many important crossover studies are controlled experiments. Crossover designs are common for experiments in many scientific disciplines, for example psychology, education, pharmaceutical science, and health care, especially medicine. Randomized, controlled, crossover experiments are especially important in health care. In a randomized clinical trial, the subjects are randomly assigned treatments. When such a trial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adaptive Design (medicine)
In an adaptive design of a clinical trial, the parameters and conduct of the trial for a candidate approved drug, drug or vaccine may be changed based on an interim analysis. Adaptive design typically involves advanced statistics to interpret a clinical trial clinical endpoint, endpoint. This is in contrast to traditional single-arm (i.e. non-randomized) clinical trials or Randomized controlled trial, randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process takes place at certain points in the trial, prescribed in the trial protocol. Importantly, this trial protocol is set before the trial begins with the adaptation schedule and processes specified. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. The PANDA (A Practical Adaptive & Novel Designs and Analysis toolkit) provides not only a summary of diffe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |