|
Cyanohydrins
In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is , where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst: : In this reaction, the nucleophilic ion attacks the electrophilic carbonyl carbon in the ketone, followed by protonation by HCN, thereby regenerating the cyanide anion. Cyanohydrins are also prepared by displacement of sulfite by cyanide salts: : Cyanohydrins are intermediates in the Strecker amino acid synthesis. In aqueous acid, they are hydrolyzed to the α-hydroxy acid. Acetone cyanohydrins Acetone cyanohydrin, (CH3)2C(OH)CN is the cya ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanide Anion
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms. In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom. In Inorganic compound, inorganic cyanides, the cyanide group is present as the anion . Soluble Salt (chemistry), salts such as sodium cyanide (NaCN) and potassium cyanide (KCN) are highly toxic. Hydrocyanic acid, also known as hydrogen cyanide, or HCN, is a highly Volatility (chemistry), volatile liquid that is produced on a large scale industrially. It is obtained by acidification of cyanide salts. Organic compound, Organic cyanides are usually called nitriles. In nitriles, the group is linked by a covalent bond to carbon. For example, in acetonitrile (), the cyanide group is bonded to methyl (). Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus rather ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms. In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom. In inorganic cyanides, the cyanide group is present as the anion . Soluble salts such as sodium cyanide (NaCN) and potassium cyanide (KCN) are highly toxic. Hydrocyanic acid, also known as hydrogen cyanide, or HCN, is a highly volatile liquid that is produced on a large scale industrially. It is obtained by acidification of cyanide salts. Organic cyanides are usually called nitriles. In nitriles, the group is linked by a covalent bond to carbon. For example, in acetonitrile (), the cyanide group is bonded to methyl (). Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus rather toxic. Bonding The cyanide ion is isoelectronic with carbon m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanohydrin Reaction
A cyanohydrin reaction is an organic chemical reaction in which an aldehyde or ketone reacts with a cyanide anion or a nitrile to form a cyanohydrin. This nucleophilic addition is a reversible reaction but with aliphatic carbonyl compounds equilibrium is in favor of the reaction products. The cyanide source can be potassium cyanide, sodium cyanide or trimethylsilyl cyanide. With aromatic aldehydes such as benzaldehyde, the benzoin condensation is a competing reaction. The reaction is used in carbohydrate chemistry as a chain extension method for example that of D-xylose. Examples Reaction mechanism Asymmetric synthesis The asymmetric cyanohydrin reaction of benzaldehyde with trimethylsilylcyanide is made possible by employment of (R)- Binol at 1–10% catalyst loading. This ligand firsts reacts with a lithium alkoxy compound to form a lithium binaphtholate Complex. The chemist Urech in 1872 was the first to synthesize cyanohydrins from ketones with alkali cya ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanation Of Aldehyde With Bisulfate
In organic synthesis, cyanation is the attachment or substitution of a cyanide group on various substrates. Such transformations are high-value because they generate C-C bond. Furthermore nitriles are versatile functional groups. Cyanation to form sp3 nitriles Typically, alkyl nitriles are formed ''via'' SN1 or SN2-type cyanation with alkyl electrophiles. Illustrative is the synthesis of benzyl cyanide by the reaction of benzyl chloride and sodium cyanide. In some cases cuprous cyanide is used instead of sodium cyanide. Cyanation of ketones or aldehydes yields the corresponding cyanohydrins, which can be done directly with the cyanide ion (the cyanohydrin reaction) or by using bisulfite, followed by displacement of sulfite: A related reaction is hydrocyanation, which installs the elements of H-CN. Cyanation of arenes Cyanation of arenes offers access to benzoic acid derivatives, as well as the utility of aryl nitriles themselves in as fine chemicals: A variety of mechanisti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully. Solids Many salts and solids can be dried using heat, or under vacuum. Desiccators can also be used to store reagents in dry conditions. Common desiccants include phosphorus pentoxide and silica gel. Chemists may also require dry glassware for sensitive reactions. This can be achieved by drying glassware in an oven, by flame, or under vacuum. Dry solids can be produced by freeze-drying, which is also known as lyophilization. Liquids or solvents In many cases, the presence of water can prevent a reaction from happening, or cause undesirable products to form. To prevent this, anhydrous solvents must be used when perfor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Hydride
Lithium hydride is an inorganic compound with the formula Li H. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic of a salt-like (ionic) hydride, it has a high melting point, and it is not soluble but reactive with all protic organic solvents. It is soluble and nonreactive with certain molten salts such as lithium fluoride, lithium borohydride, and sodium hydride. With a molar mass of 7.95 g/mol, it is the lightest ionic compound. Physical properties LiH is a diamagnetic and an ionic conductor with a conductivity gradually increasing from at 443 °C to 0.18 Ω−1cm−1 at 754 °C; there is no discontinuity in this increase through the melting point. The dielectric constant of LiH decreases from 13.0 (static, low frequencies) to 3.6 (visible-light frequencies). LiH is a soft material with a Mohs hardness of 3.5. Its compressive creep (per 100 hours) rapidly increases from 100% at 475 °C, meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arene
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell. Heteroarenes are closely related, since at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are called aliphatic. Benzene ring model Benzene, C6H6, is the least complex aromatic hydrocarbon, and it was the first one named as such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
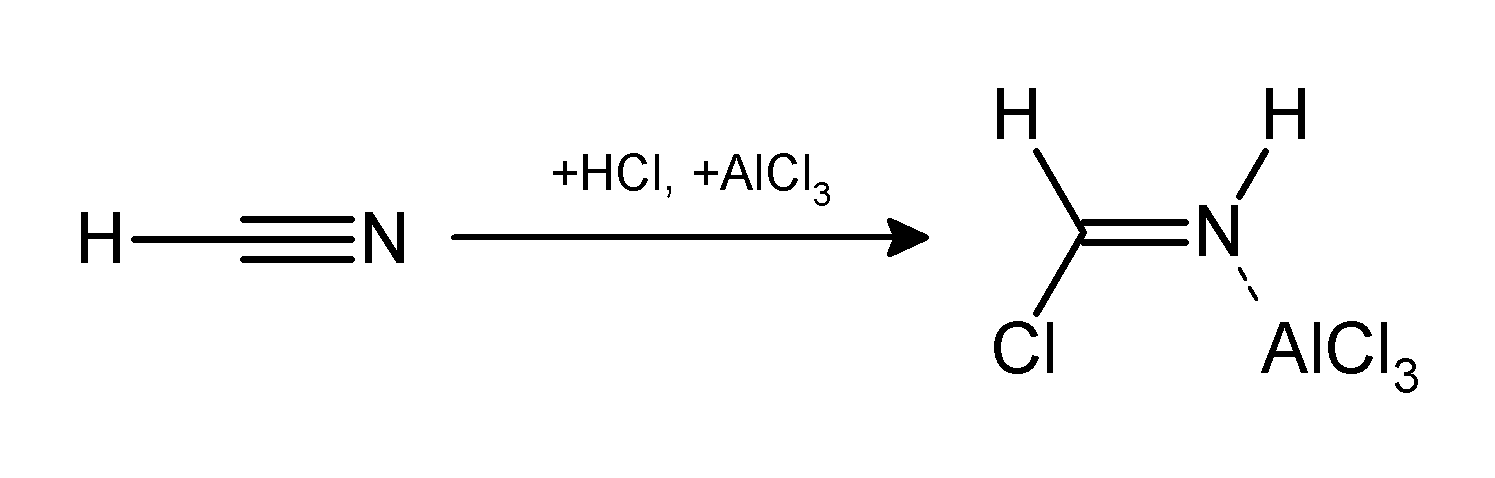
Gattermann-Koch Reaction
The Gattermann reaction, (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as AlCl3. It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction. Modifications have shown that it is possible to use sodium cyanide or cyanogen bromide in place of hydrogen cyanide. The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide. Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN. The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(Cl)2 that serves as the Lewis-acid catalyst ''in-situ''. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene. Gattermann–Koch reaction The Gattermann–Koch reaction, named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Methacrylate
Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA). Production and properties Given the scale of production, many methods have been developed starting from diverse two- to four-carbon precursors.. Two principal routes appear to be commonly practiced. Cyanohydrin route The compound is manufactured by several methods, the principal one being the acetone cyanohydrin (ACH) route. ACH is produced by condensation of acetone and hydrogen cyanide. The cyanohydrin is hydrolyzed in the presence of sulfuric acid to a sulfate ester of the methacrylamide. Methanolysis of this ester gives ammonium bisulfate and MMA. Although widely used, the ACH route coproduces substantial amounts of ammonium sulfate. :(CH3)2CO + HCN → (CH3)2C(OH)CN :(CH3)2C(OH)CN + H2SO4 → (CH3)2C(OSO3H)C(O)N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |