|
Corilagin
Corilagin is an ellagitannin. Corilagin was first isolated in 1951 from Dividivi extract and from '' Caesalpinia coriaria'', hence the name of the molecule. It can also be found in '' Alchornea glandulosa'' and in the leaves of ''Punica granatum'' (pomegranate). It is a weak carbonic anhydrase inhibitor. Ellagic acid and corilagin inhibit TGF-β1–dependent EMT and has been shown to attenuate fibrogenesis in a mouse model. Fibrosis is also indicated in many health conditions, including skin aging and MRSA Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. ... susceptibility. References Ellagitannins Pomegranate ellagitannins {{Aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesalpinia Coriaria
''Libidibia coriaria'', synonym ''Caesalpinia coriaria'', is a leguminous tree or large shrub native to the Caribbean, Central America, Mexico, and northern and western South America. Common names include divi-divi, cascalote, guaracabuya, guatapana, nacascol, tan yong, and ''watapana'' ( Aruba). Description ''L. coriaria'' rarely reaches its maximum height of because its growth is contorted by the trade winds that batter the exposed coastal sites where it often grows. In other environments it grows into a low dome shape with a clear sub canopy space. Leaves are bipinnate, with 5–10 pairs of pinnae, each pinna with 15–25 pairs of leaflets; the individual leaflets are 7 mm long and 2 mm broad. The fruit is a twisted pod long. Taxonomy The species was first described by Nikolaus Joseph von Jacquin in 1763, as ''Poinciana coriaria''. In 1799, Carl Ludwig Willdenow transferred it to the genus ''Caesalpinia'', and in 1830, Diederich von Schlechtendal transfe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alchornea Glandulosa
''Alchornea glandulosa'' is a tree species of the Acalyphoideae native to South America, growing in southern Brazil from Minas Gerais to Rio Grande do Sul. It is locally known as ''tamanqueiro'', ''tapiá'' or ''amor seco''. This gnarled tree grows preferentially in riparian forest, where it a common pioneer species growing to a height of 10–20 m. It is essentially evergreen, though in the hot austral summer months there is a more pronounced changeover of leaves, and branches are denuded to some extent. The fruit is about 8.7 mm long by 5.9 mm wide on average, and contains one round seed measuring about 4.45 mm in diameter; very rarely a second seed develops. This sticks out of an aril at the fruit's tip; when ripe, the seedcoat turns bright red and the fruit somewhat resembles that of a yew with a larger and more prominent seed. Fruit ripen in the summer months, roughly between September/October and December/January in S Brazil, and as the trees bear less leave ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galloyl
Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6 H2( OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates". Isolation and derivatives Gallic acid is easily freed from gallotannins by acidic or alkaline hydrolysis. When heated with concentrated sulfuric acid, gallic acid converts to rufigallol. Hydrolyzable tannins break down on hydrolysis to give gallic acid and glucose or ellagic acid and glucose, known as gallotannins and ellagitannins, respectively. Biosynthesis Gallic acid is formed from 3-dehydroshikimate by the action of the enzyme shikimate dehydrogenase to produce 3,5-didehydroshikimate. This latter compound aromatizes. Reactions Oxidation and oxidative coupling Alkaline solutions of gall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ellagitannin
The ellagitannins are a diverse class of hydrolyzable tannins, a type of polyphenol formed primarily from the oxidative linkage of galloyl groups in 1,2,3,4,6-pentagalloyl glucose. Ellagitannins differ from gallotannins, in that their galloyl groups are linked through C-C bonds, whereas the galloyl groups in gallotannins are linked by depside bonds. Ellagitannins contain various numbers of hexahydroxydiphenoyl units, as well as galloyl units and/or sanguisorboyl units bounded to sugar moiety. In order to determine the quantity of every individual unit, the hydrolysis of the extracts with trifluoroacetic acid in methanol/water system is performed. Hexahydroxydiphenic acid, created after hydrolysis, spontaneously lactonized to ellagic acid, and sanguisorbic acid to sanguisorbic acid dilactone, while gallic acid remains intact. Ellagitannins generally form macrocycles, whereas gallotannins do not. Examples * Castalagin * Castalin * Casuarictin * Grandinin * Oeno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Punica Granatum
The pomegranate (''Punica granatum'') is a fruit-bearing deciduous shrub in the family Lythraceae, subfamily Punicoideae, that grows between tall. The pomegranate was originally described throughout the Mediterranean region. It was introduced into Spanish America in the late 16th century and into California by Spanish settlers in 1769. The fruit is typically in season in the Southern Hemisphere from March to May, and in the Northern Hemisphere from September to February. As intact sarcotestas or juice, pomegranates are used in baking, cooking, juice blends, meal garnishes, smoothies, and alcoholic beverages, such as cocktails and wine. Pomegranates are widely cultivated throughout the Middle East and Caucasus region, north and tropical Africa, Iran, Armenia, the Indian subcontinent, Central Asia, the drier parts of Southeast Asia, and the Mediterranean Basin. Etymology The name pomegranate derives from medieval Latin "apple" and "seeded". Possibly stemming from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Anhydrase Inhibitor
Carbonic anhydrase inhibitors are a class of pharmaceuticals that suppress the activity of carbonic anhydrase. Their clinical use has been established as anti-glaucoma agents, diuretics, antiepileptics, in the management of mountain sickness, gastric and duodenal ulcers, idiopathic intracranial hypertension, neurological disorders, or osteoporosis. Medical uses Carbonic anhydrase inhibitors are primarily used for the treatment of glaucoma. They may also be used to treat seizure disorder and acute mountain sickness. Because they encourage solubilization and excretion of uric acid, they can be used in the treatment of gout. Glaucoma Acetazolamide is an inhibitor of carbonic anhydrase. It is used for glaucoma, epilepsy (rarely), idiopathic intracranial hypertension, and altitude sickness. For the reduction of intraocular pressure (IOP), acetazolamide inactivates carbonic anhydrase and interferes with the sodium pump, which decreases aqueous humor formation and thus lower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ellagic Acid
Ellagic acid is a polyphenol found in numerous fruits and vegetables. It is the dilactone of hexahydroxydiphenic acid. Name The name comes from the French term ''acide ellagique'', from the word ''galle'' spelled backwards because it can be obtained from ''noix de galle'' (galls), and to distinguish it from ''acide gallique'' (gallic acid). The molecule structure resembles to that of two gallic acid molecules being assembled "head to tail" and bound together by a C–C bond (as in biphenyl, or in diphenic acid) and two lactone links (cyclic carboxylic esters). Metabolism Biosynthesis Plants produce ellagic acid from hydrolysis of tannins such as ellagitannin and geraniin. Biodegradation Urolithins are gut flora human metabolites of dietary ellagic acid derivatives. Ellagic acid has low bioavailability, with 90% remaining unabsorbed from the intestines until metabolized by microflora to the more bioavailable urolintins. History Ellagic acid was first discovered b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
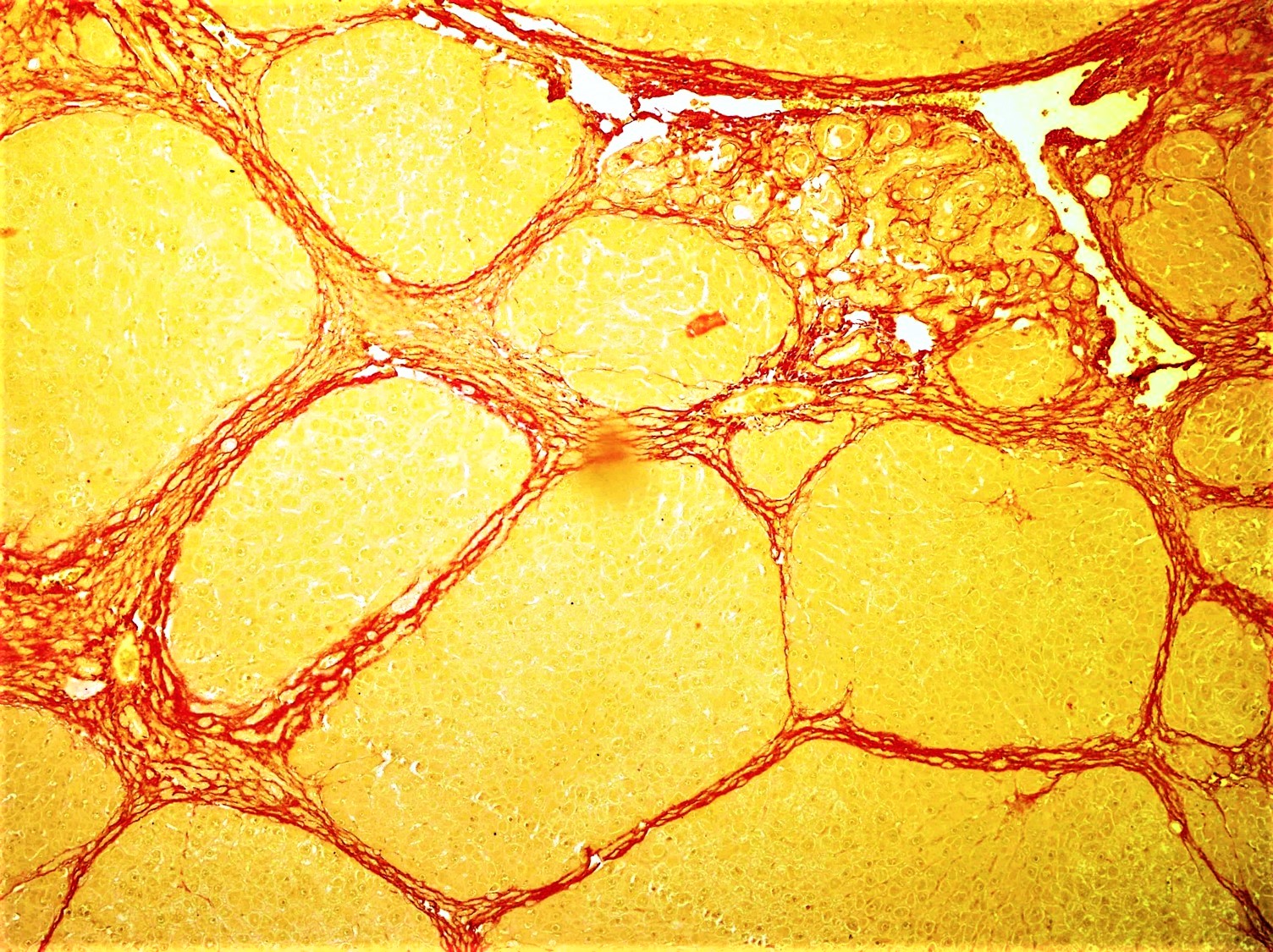
Fibrogenesis
Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of permanent scar tissue. Repeated injuries, chronic inflammation and repair are susceptible to fibrosis where an accidental excessive accumulation of extracellular matrix components, such as the collagen is produced by fibroblasts, leading to the formation of a permanent fibrotic scar. In response to injury, this is called scarring, and if fibrosis arises from a single cell line, this is called a fibroma. Physiologically, fibrosis acts to deposit connective tissue, which can interfere with or totally inhibit the normal architecture and function of the underlying organ or tissue. Fibrosis can be used to describe the pathological state of excess deposition of fibrous tissue, as well as the process of connective tissue deposition in healing. Defi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibrosis
Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of permanent scar tissue. Repeated injuries, chronic inflammation and repair are susceptible to fibrosis where an accidental excessive accumulation of extracellular matrix components, such as the collagen is produced by fibroblasts, leading to the formation of a permanent fibrotic scar. In response to injury, this is called scarring, and if fibrosis arises from a single cell line, this is called a fibroma. Physiologically, fibrosis acts to deposit connective tissue, which can interfere with or totally inhibit the normal architecture and function of the underlying organ or tissue. Fibrosis can be used to describe the pathological state of excess deposition of fibrous tissue, as well as the process of connective tissue deposition in healing. Define ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |