|
Core Charge
Core electrons are the electrons in an atom that are not valence electrons and do not participate as directly in chemical bonding. The nucleus and the core electrons of an atom form the atomic core. Core electrons are tightly bound to the nucleus. Therefore, unlike valence electrons, core electrons play a secondary role in chemical bonding and reactions by screening the positive charge of the atomic nucleus from the valence electrons. The number of valence electrons of an element can be determined by the periodic table group of the element (see valence electron): *For main-group elements, the number of valence electrons ranges from 1 to 8 (''n''s and ''n''p orbitals). *For transition metals, the number of valence electrons ranges from 3 to 12 (''n''s and (''n''−1)d orbitals). *For lanthanides and actinides, the number of valence electrons ranges from 3 to 16 (''n''s, (''n''−2)f and (''n''−1)d orbitals). All other non-valence electrons for an atom of that element are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Core
Core electrons are the electrons in an atom that are not valence electrons and do not participate as directly in chemical bonding. The atomic nucleus, nucleus and the core electrons of an atom form the atomic core. Core electrons are tightly bound to the nucleus. Therefore, unlike valence electrons, core electrons play a secondary role in chemical bonding and reactions by screening the positive charge of the atomic nucleus from the valence electrons. The number of valence electrons of an element can be determined by the group (periodic table), periodic table group of the element (see valence electron): *For main-group elements, the number of valence electrons ranges from 1 to 8 (''n''s and ''n''p orbitals). *For transition metals, the number of valence electrons ranges from 3 to 12 (''n''s and (''n''−1)d orbitals). *For lanthanides and actinides, the number of valence electrons ranges from 3 to 16 (''n''s, (''n''−2)f and (''n''−1)d orbitals). All other non-valence electrons fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by the nuclei and all the other electrons. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, a level of energy is associated with each electron configuration. In certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First Ionization Energy
In physics and chemistry, ionization energy (IE) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. The first ionization energy is quantitatively expressed as :X(g) + energy ⟶ X+(g) + e− where X is any atom or molecule, X+ is the resultant ion when the original atom was stripped of a single electron, and e− is the removed electron. Ionization energy is positive for neutral atoms, meaning that the ionization is an endothermic process. Roughly speaking, the closer the outermost electrons are to the nucleus of the atom, the higher the atom's ionization energy. In physics, ionization energy (IE) is usually expressed in electronvolts (eV) or joules (J). In chemistry, it is expressed as the energy to ionize a mole of atoms or molecules, usually as kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Comparison of ionization energies of atoms in the periodic table reveals two periodic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Trend
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain elements when grouped by period and/or group. They were discovered by the Russian chemist Dmitri Mendeleev in 1863. Major periodic trends include atomic radius, ionization energy, electron affinity, electronegativity, nucleophilicity, electrophilicity, valency, nuclear charge, and metallic character. Mendeleev built the foundation of the periodic table. Mendeleev organized the elements based on atomic weight, leaving empty spaces where he believed undiscovered elements would take their places. Mendeleev's discovery of this trend allowed him to predict the existence and properties of three unknown elements, which were later discovered by other chemists and named gallium, scandium, and germanium. English physicist Henry Moseley discovered that organizing the elements by atomic number instead of atomic weight would naturally group elements with si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Four widely used definitions of atomic radius are: Van der Waals radius, ionic radius, metallic radius and covalent radius. Typically, because of the difficulty to isolate atoms in order to measure their radii separately, atomic radius is measured in a chemically bonded state; however theoretical calculations are simpler when considering atoms in isolation. The dependencies on environment, probe, and state lead to a multiplicity of definitions. Depending on the definition, the term may apply to atoms in condensed matter, covalently bonding in molecules, or in ionized and excited states; and its value may be obtained through experimental measurements, or computed from theore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
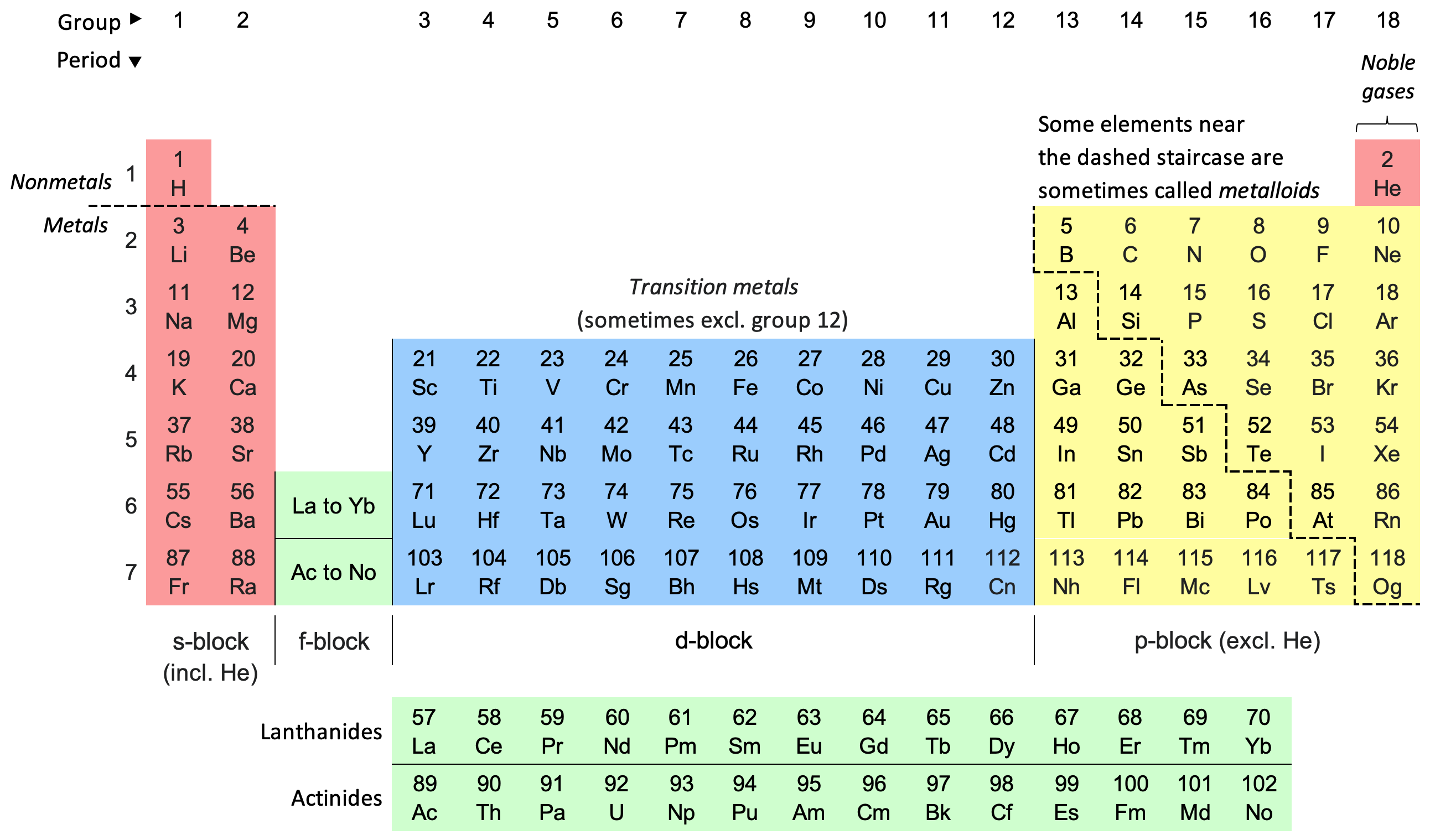
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactive decay, radioactively decays, usually by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes of uranium, isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordial nuclide, primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few Parts-per notation#Parts-per expressions, parts per million in soil, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |