|
Copper(I) Hydroxide
Copper(I) hydroxide is the inorganic compound with the chemical formula of CuOH. Little evidence exists for its existence. A similar situation applies to the monohydroxides of gold(I) and silver(I). Solid CuOH has been claimed however as an unstable yellow-red solid. The topic has been the subject of theoretical analysis. Copper(I) hydroxide would also be expected to easily oxidise to copper(II) hydroxide: : It would also be expected to rapidly dehydrate: : Solid CuOH would be of interest as a possible intermediate in the formation of copper(I) oxide (Cu2O), which has diverse applications, e.g. applications in solar cells. Solid CuOH Theoretical calculations predict that CuOH would be stable. Specifically, the dissociation of Cu(OH)2− leading to CuOH is subject to an energy of 62 ± 3 kcal/mol. : Without evidence for its existence, CuOH has been invoked as a catalyst in organic synthesis Gaseous CuOH Gaseous CuOH has been characterized spectroscopically using intracav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Hydroxide
Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist of a mixture of copper(II) carbonate and hydroxide. Cupric hydroxide is a strong base, although its low solubility in water makes this hard to observe directly. Occurrence Copper(II) hydroxide has been known since Smelting#Copper and bronze, copper smelting began around 5000 BC although the alchemy, alchemists were probably the first to manufacture it by mixing solutions of lye (sodium or potassium hydroxide) and blue vitriol (copper(II) sulfate). Sources of both compounds were available in antiquity. It was produced on an industrial scale during the 17th and 18th centuries for use in pigments such as blue verditer and Bremen green. These pigments were used in ceramics (art), ceramics and painting. Mineral The mineral of the formula Cu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Oxide
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula . It is one of the principal oxides of copper, the other being copper(II) oxide or cupric oxide (CuO). The compound can appear either yellow or red, depending on the size of the particles. Cuprous oxide is found as the mineral cuprite. It is a component of some antifouling paints, and has other applications including some that exploit its property as a semiconductor. Preparation Copper(I) oxide may be produced by several methods. Most straightforwardly, it arises via the oxidation of copper metal: : Additives such as water and acids affect the rate as well as the further oxidation to copper(II) oxides. It is also produced commercially by reduction of copper(II) solutions with sulfur dioxide. Alternatively, it may be prepared via the reduction of copper(II) acetate with hydrazine: : Copper(I) chloride solutions react with base to give the same material. In all cases, the color of the cuprous oxide i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laser Spectroscopy
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and electronic structure of matter to be investigated at the atomic, molecular and macro scale, and over astronomical distances. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Current applications of spectroscopy include biomedical spectroscopy in the areas of tissue analysis and medical imaging. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CSD File AKONAQ
CSD may refer to: Finance * Central securities depository * Confederate States Dollar * Serbian dinar, by previous ISO 4217 code Organizations Education * California School for the Deaf (other), several institutions * Canyons School District, in Utah, US * Cheltenham School District, in Pennsylvania, US * Christina School District, in Delaware, US * Cleveland School District, in Mississippi, US * Cordova School District, in Alaska, US Other organizations * Canteen Stores Department (India), a chain of stores operated by the Indian Ministry of Defence at military bases * CSD Pakistan (Canteen Stores Department), a chain of stores operated by the Pakistani Ministry of Defence * Chartered Society of Designers, a British learned society for various kinds of design work * Commission on Sustainable Development (1992–2013), a former UN agency * Communication Service for the Deaf, an American non-profit company providing ASL services * Congress of Democratic Trade Un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectrometer
A spectrometer () is a scientific instrument used to separate and measure Spectrum, spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. In visible light a spectrometer can separate white light and measure individual narrow bands of color, called a spectrum. A mass spectrometer measures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers were History_of_spectroscopy, developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine Analytical_chemistry#Spectroscopy, chemical composition drove its advancement and continues to be one of its primary uses. Spectrometers are used in Astronomical spectroscopy, astronomy to analyze the chemical composition of Astronomical_spe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
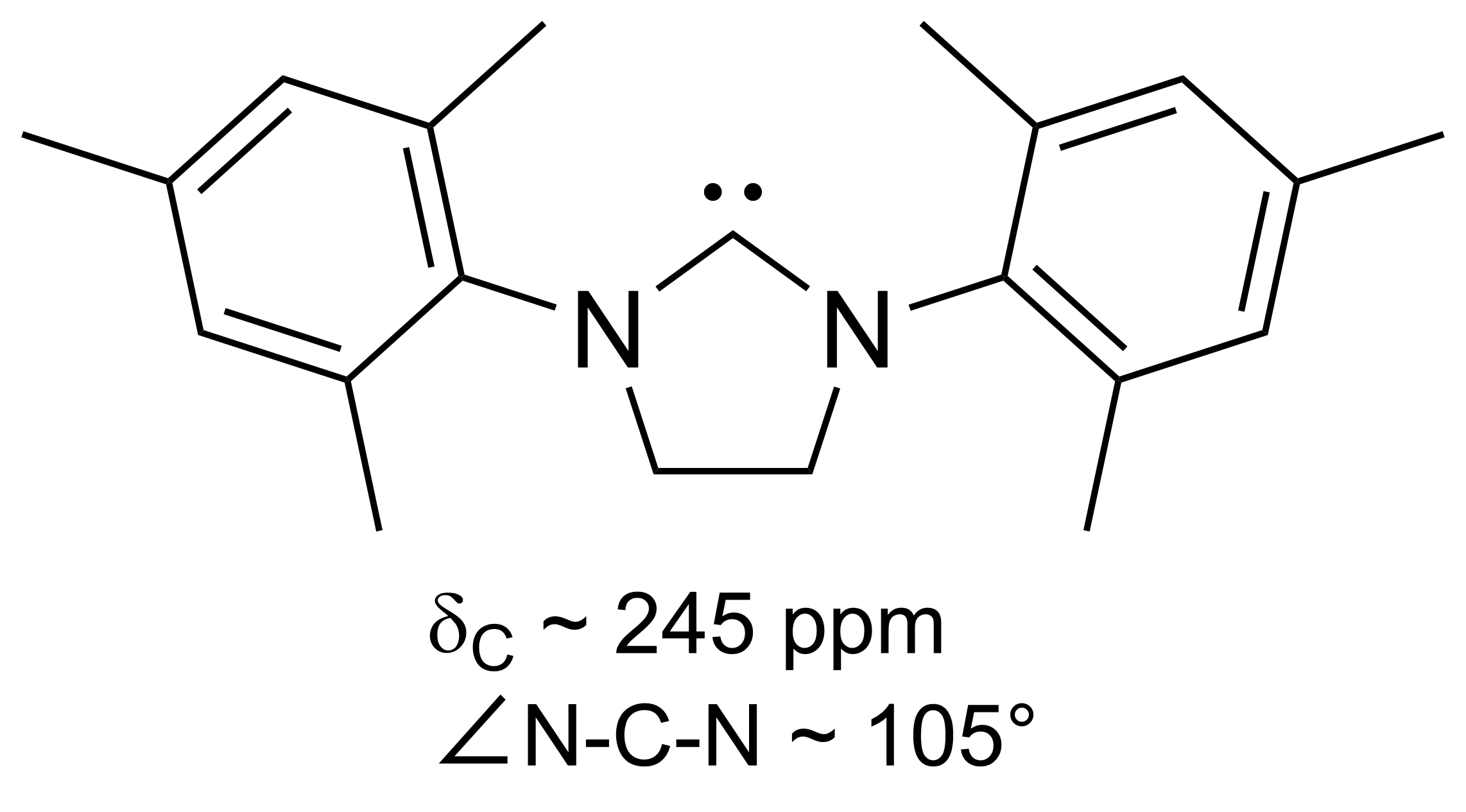
Persistent Carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), in which nitrogen atoms flank the formal carbene. Modern theoretical analysis suggests that the term "persistent carbene" is in fact a misnomer. Persistent carbenes do not in fact have a carbene electronic structure in their ground state, but instead an ylide stabilized by Aromaticity, aromatic resonance or steric shielding. Excitation to a carbene structure then accounts for the carbene-like dimerization that some persistent carbenes undergo over the course of days. Persistent carbenes in general, and Arduengo carbenes in particular, are popular ligands in organometallic chemistry. Histor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aquo Complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry . Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand (" homoleptic aquo complexes"), but of course many complexes are known to consist of a mix of aquo and other ligands. Stoichiometry and structure Hexa-aquo complexes Most aquo complexes are mono-nuclear, with the general formula , with or 3; they have an octahedral structure. The water molecules function as Lewis bases, donating a pair of electrons to the metal ion and forming a dative covalent bond with it. Typical examples are listed in the following table. Tutton's salts are crystalline compounds with the generic formula (where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Compounds
Copper is a chemical element; it has Chemical symbol, symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductility, ductile metal with very high thermal conductivity, thermal and electrical conductivity. A freshly exposed surface of pure copper has a Copper (color), pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material#Metal, building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable, unalloyed metallic form. This means that copper is a native metal. This led to very early human use in several regions, from . Thousands of years later, it was the first metal to be Smelting, smelted from sulfide ores, ; the first metal to be cast into a shape in a mold, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |